June 16, 2024 By Sadia Khan 5 minutes read
Heat transfer is a fundamental concept that affects our daily lives in many ways. From keeping our homes warm in winter to cooling electronic devices, understanding how heat transfer happens is crucial for various industries.
Professionals in fields like engineering, physics, and environmental science rely on a deep understanding of heat transfer mechanisms to design efficient systems and solve real-world problems. In this article, we will discuss in detail heat transfer and conduction, convection, and radiation.
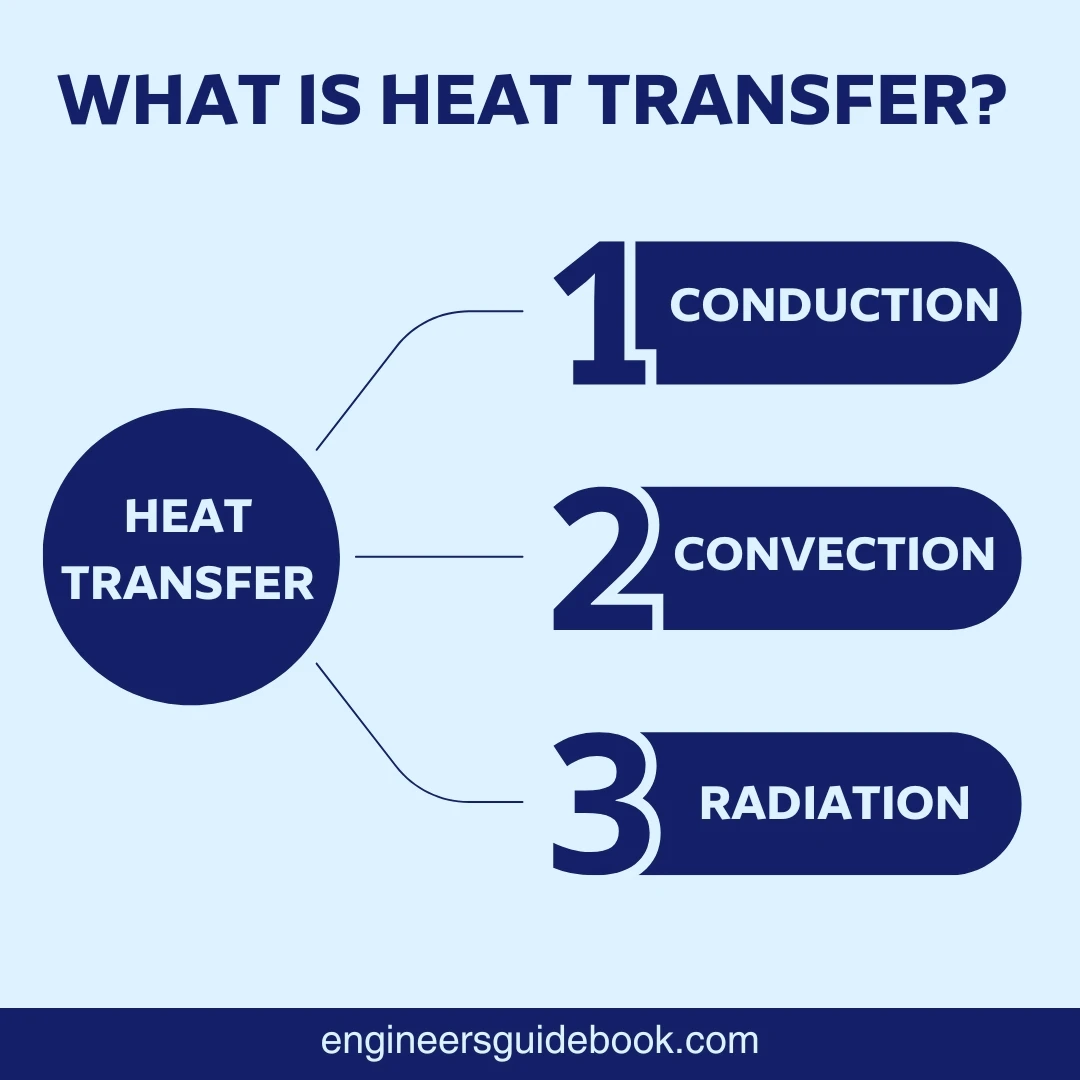
There are three primary modes through which heat transfers:
Conduction
Convection
Radiation
Each mode plays a distinct role in how heat moves from one place to another, whether it’s through direct contact, fluid motion, or electromagnetic waves.
Heat transfer refers to the movement of thermal energy from one object or substance to another due to a temperature difference. This energy transfer occurs until thermal equilibrium, where temperatures equalize.
The basic laws of thermodynamics first law of thermodynamics and the second law of thermodynamics also guide heat transfer processes.
1st Law of thermodynamics also known as the law of conservation of energy states that:
Energy cannot be created or destroyed in a process. When heat is added to a system, it either increases the internal energy of the system or is used to do work. The total energy remains constant.
2nd Law of thermodynamics in terms of heat transfer is stated as “
Heat naturally flows from hotter to colder objects, never spontaneously from colder to hotter objects. This law also states that the total entropy (a measure of disorder or randomness) of an isolated system tends to increase over time.
These laws explain how heat moves as well energy can’t disappear (only change from one form to another ), and heat flows from hot to cold areas. They help us understand and improve how heat is transferred in the real world.
Conduction is the heat transfer process through a material due to direct contact between particles.
When one part of a material gets hot, its molecules gain kinetic energy and vibrate, passing it to neighboring molecules. This process continues until the heat spreads throughout the material.
Fourier’s Law of Heat Conduction describes how heat conducts through a material:
Q = −kA dx/dT
where:
This equation shows that the heat transfer rate is proportional to the temperature difference and the material’s ability to conduct heat.
Thermal conductivity is a measure of how well a material conducts heat. Materials with high thermal conductivity, like metals, transfer heat quickly. Those with low thermal conductivity, like insulators, transfer heat more slowly.
Factors affecting thermal conductivity include the material’s composition, density, and temperature.
Conduction is crucial in many engineering and technological applications:
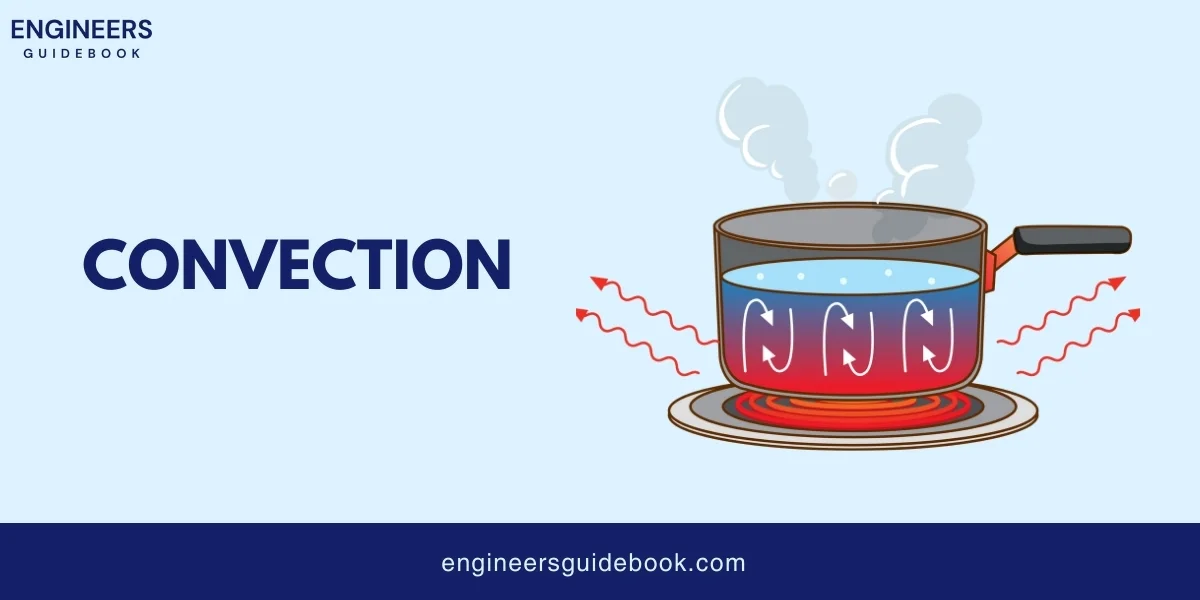
Convection is the transfer of heat by the movement of a fluid, which can be a liquid or a gas.
When a fluid is heated, it becomes less dense and rises, while cooler fluid sinks, creating a cycle that transfers heat. There are two types of convection:
To measure convection in real-world applications, we use Newton’s law of cooling, which states that :
The rate of heat loss of a body is directly proportional to the difference in temperature between the body and its surroundings, provided the temperature difference is not too large.
In simpler terms, an object will cool down faster if the temperature difference between the object and its environment is greater.
Q=hA(Ts−Tf)
where:
This equation shows that the heat transfer rate depends on the temperature difference, the surface area, and the convection coefficient.
The convection heat transfer coefficient ( h ) measures how effectively a fluid transfers heat by convection. Factors affecting this coefficient include the fluid’s properties, flow velocity, and surface roughness.
Engineers calculate the convection heat transfer coefficient ( h ) using empirical correlations based on experimental data and fluid dynamics principles.
Convection is essential in various engineering and technological applications:
Radiation is the transfer of heat through electromagnetic waves. Unlike conduction and convection, it doesn’t require a medium to travel through. This means heat can move through empty space, like the heat from the sun reaching Earth.
Thermal radiation occurs when an object emits electromagnetic waves due to its temperature. These waves can be in the form of infrared radiation, visible light, or even microwaves.
The Stefan-Boltzmann Law states that the total radiant heat energy emitted per unit surface area of a black body is directly proportional to the fourth power of its absolute temperature.
This law is crucial in understanding thermal radiation and heat transfer.
E=σT4
This law shows that the amount of heat radiated increases rapidly as the temperature rises.
A perfect black body is an idealized physical body that absorbs all incident electromagnetic radiation and re-emits it perfectly according to this law.
Emissivity is a measure of how effectively a surface emits thermal radiation compared to a perfect black body. It ranges from 0 to 1.
A high emissivity means the material is good at radiating heat, while a low emissivity indicates poor radiation. Factors affecting emissivity include the material’s surface properties and temperature.
Radiation is vital in various applications:
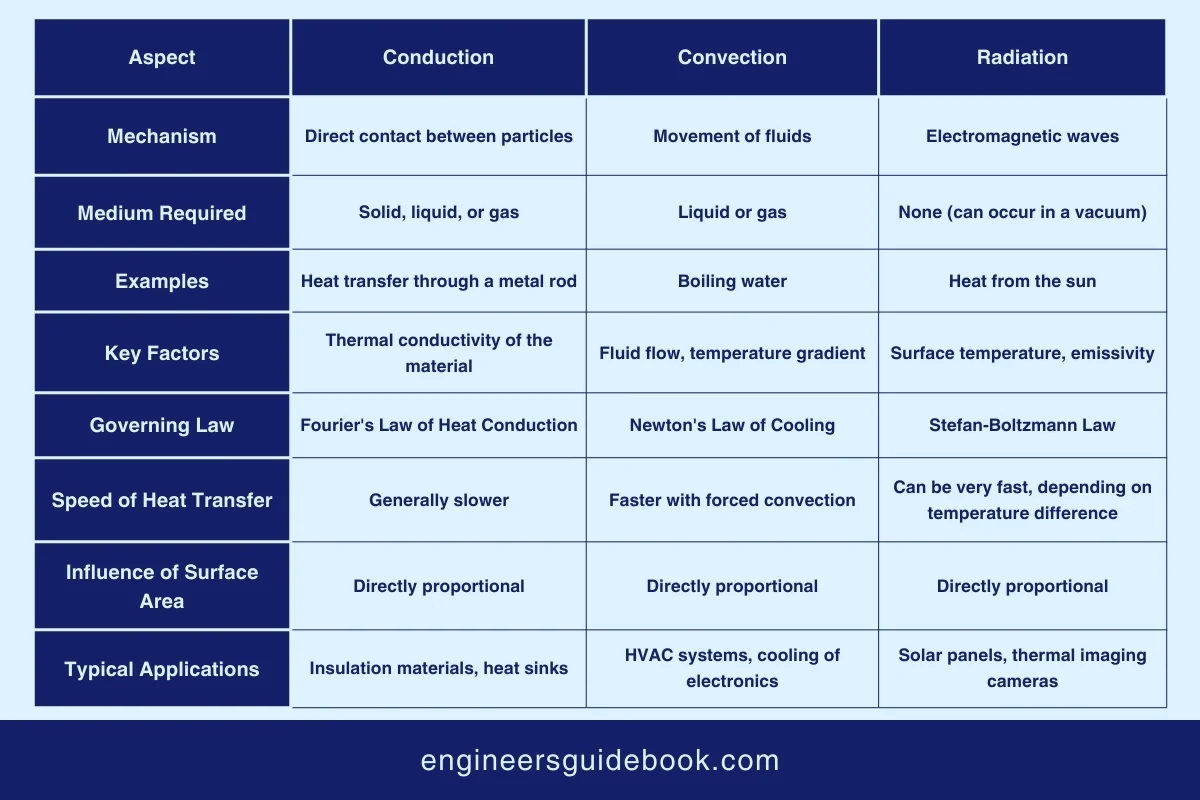
Below given table is showing a comparison between conduction, convection, and radiation, highlighting their distinct mechanisms of heat transfer
Heat transfer becomes more intricate in complex systems where multiple factors influence how heat moves. These systems often involve combinations of conduction, convection, and radiation.
In thermal power plants, for example, heat from burning fuels (radiation) heats water in boilers through conduction, and the resulting steam transfers heat through convection to turbines that generate electricity.
Solar panels, another example, convert sunlight (radiation) into electricity through photovoltaic cells, managing heat transfer to maximize energy conversion efficiency.
Recent advancements in heat transfer technology have brought about new materials and methods that enhance efficiency and sustainability across various applications:
Nanomaterials: Scientists are exploring nanotechnology to develop materials with unique properties that improve heat transfer. Nanomaterials can conduct heat efficiently while being lightweight and durable. They have applications in electronics cooling, aerospace, and renewable energy systems like solar panels.
Phase Change Materials (PCMs): These materials store and release thermal energy during phase transitions (like solid to liquid).PCMs help regulate temperatures effectively in buildings and vehicles, reducing energy consumption for heating and cooling.
Smart Materials: These materials can change their properties in response to external stimuli like temperature changes. For heat transfer, smart materials adjust conductivity or reflectivity to optimize thermal management. They are being developed for applications in adaptive building facades and wearable technology.
Looking ahead, future innovations in heat transfer technology are expected to focus on:
Enhanced Energy Efficiency: Continued efforts will be made to improve the efficiency of heat transfer processes, reducing energy consumption and environmental impact.
Integration with Renewable Energy: Heat transfer technology will play a crucial role in integrating renewable energy sources like solar and geothermal into mainstream applications.
Advancements in Thermal Management: Innovations will target better control and management of thermal loads in electronics, vehicles, and industrial processes, ensuring reliability and performance.
Heat transfer is essential for everything from cooking meals to powering spacecraft. We’ve explored how heat moves through conduction, convection, and radiation.
Each plays a unique role in our daily lives and industries. Looking ahead, advancements in technology like nanomaterials and smart materials offer exciting possibilities for improving how we manage heat.
By continuing to innovate and stay informed, we can create a more efficient and sustainable world, tackling challenges like energy efficiency and climate change head-on through better heat transfer solutions.
There are three primary modes through which heat transfers:
Conduction
Convection
Radiation
Each mode plays a distinct role in how heat moves from one place to another
Conduction: Conduction is the transfer of heat through a material or between two materials that are in direct contact, due to the collision of molecules within the material.
Convection: Convection is the transfer of heat through the movement of fluids (liquids or gases), involving circulation due to temperature differences.
Conduction: Conduction is the transfer of heat through a material or between two materials that are in direct contact, due to the collision of molecules within the material.
Convection: Convection is the transfer of heat through the movement of fluids (liquids or gases), involving circulation due to temperature differences.
Radiation: Radiation is the transfer of heat through electromagnetic waves, occurring without the need for a medium and based on the temperature of emitting objects.
Heat is transferred by convection through the movement of fluids (liquids or gases). When a fluid is heated, its particles gain kinetic energy and become less dense, causing them to rise.
Cooler, denser fluid then moves in to take its place, creating a continuous cycle of circulation that transfers heat from hotter regions to cooler ones.
The Stefan-Boltzmann Law states that the total radiant heat energy emitted per unit surface area of a black body is directly proportional to the fourth power of its absolute temperature.
This law is crucial in understanding thermal radiation and heat transfer.
E=σT4
Convection: Convection is the transfer of heat through the movement of fluids (liquids or gases), involving circulation due to temperature differences.
Conduction: Conduction is the transfer of heat through a material or between two materials that are in direct contact, due to the collision of molecules within the material.
Radiation: Radiation is the transfer of heat through electromagnetic waves, occurring without the need for a medium and based on the temperature of emitting objects.
Examples of radiation include sunlight warming the Earth’s surface, heat emitted from a glowing fireplace, and infrared radiation used in thermal imaging cameras to detect heat signatures.
Thermal conductivity is a measure of how well a material conducts heat. Materials with high thermal conductivity, like metals, transfer heat quickly. Those with low thermal conductivity, like insulators, transfer heat is more slowly.
Factors affecting thermal conductivity include the material’s composition, density, and temperature.
Emissivity is a measure of how effectively a surface emits thermal radiation compared to a perfect black body. It ranges from 0 to 1.
A high emissivity means the material is good at radiating heat, while a low emissivity indicates poor radiation. Factors affecting emissivity include the material’s surface properties and temperature.
Boiling water involves both conduction and convection. The heat from the stove (conduction) warms the bottom of the pot, transferring energy directly to the water molecules.
As the water heats, convection currents form: hotter water rises while cooler water sinks, creating circulation that spreads heat throughout the pot and causes the water to boil.
A real-life example of convection is the movement of warm air rising and cooler air sinking in a room heated by a radiator.
As the radiator warms the air next to it, the warm air expands and becomes less dense, causing it to rise toward the ceiling.
Meanwhile, cooler air from other parts of the room moves in to replace it, creating a convection current that circulates heat throughout the space.

Sadia Khan, a Chemical Engineering PhD graduate from Stanford University, focuses on developing advanced materials for energy storage. Her pioneering research in nanotechnology and battery technology is setting new standards in the field.
Explore the Engineer’s Guidebook! Find the latest engineering tips, industry insights, and creative projects. Get inspired and fuel your passion for engineering.
© 2023-2024 Engineer’s Guidebook. All rights reserved. Explore, Innovate, Engineer.