1. Introduction
Thermodynamics governs the behavior of energy and its transformations across all forms of matter. Whether it’s the boiling of water, the combustion in an engine, or the metabolism in a living cell, thermodynamic principles dictate how energy is conserved, transferred, and degraded.
It’s a field that bridges the microscopic motion of particles with macroscopic phenomena, offering clarity to processes both familiar and arcane. Understanding thermodynamics is crucial to deciphering not just the mechanics of machines but also the subtle elegance of nature’s equilibrium.
2. What Are the Laws of Thermodynamics?
The laws of thermodynamics are foundational tenets of physical science that outline how energy flows and changes within a system. These principles aren’t merely theoretical—they’re universal constraints that apply to everything from celestial bodies to quantum particles.
Comprising four distinct laws, they collectively describe how heat and energy interact with matter, setting the limits of what is energetically possible in any process.
2.1 Why Thermodynamics Is Central to Physics and Everyday Life
Thermodynamics is not limited to laboratories or academic textbooks; it permeates every facet of life. It governs how refrigerators preserve food, how engines convert fuel into motion, and how the Earth’s climate system operates.
In physics, thermodynamics provides a critical lens through which energy transformations and entropy are analyzed. It enables engineers to design efficient machines and helps biologists understand cellular respiration.
Its relevance spans disciplines, making it one of the most interdisciplinary and indispensable areas of science.
3. A Brief History of Thermodynamics
The emergence of thermodynamics was not born from abstraction but from necessity—specifically, the need to improve the efficiency of early steam engines.
What began as a practical concern evolved into a robust scientific framework that underpins vast areas of modern technology and theory.
3.1 From Steam Engines to Quantum Systems
The origins of thermodynamic thought trace back to the Industrial Revolution, where the optimization of steam engines demanded a better understanding of heat and energy. As steam technology advanced, so too did the need for theoretical foundations.
Over time, this empirical pursuit matured into a formal science, expanding its reach from piston-and-cylinder mechanics to the statistical behavior of atomic particles.
3.2 Key Scientists Who Shaped Thermodynamic Theory
Several towering intellects played pivotal roles in codifying thermodynamic laws. Sadi Carnot introduced the concept of the ideal heat engine, laying the groundwork for efficiency theory.
Rudolf Clausius and William Thomson (Lord Kelvin) formalized the laws of energy conservation and entropy. James Joule’s experiments revealed the equivalence of heat and work, cementing the first law.
Later, Ludwig Boltzmann linked entropy to molecular behavior, introducing statistical mechanics and deepening our grasp of disorder and probability.
These contributions, scattered across decades and disciplines, coalesced into a coherent and enduring doctrine.
4. Understanding Energy
Energy is the lifeblood of thermodynamic study. Without a clear grasp of energy in its manifold forms, the laws of thermodynamics lose their anchoring relevance.
Energy is both conserved and transformed—it can neither be created nor destroyed, but it can shift forms, sometimes in unexpected ways.
4.1 The Different Forms of Energy
Energy manifests in kinetic motion, potential positioning, chemical bonds, electromagnetic waves, and thermal agitation. Kinetic energy reflects motion; potential energy stores the capacity for action. Chemical energy resides in molecular bonds, while nuclear energy emerges from atomic nuclei.
Electrical and magnetic energies ripple through space via charged particles and fields. Each form of energy can morph into another, governed by thermodynamic constraints. This versatility underlies the operation of everything from hydroelectric dams to photosynthetic cells.
4.2 The Concept of Work and Heat Transfer
Work and heat are the two primary modalities through which energy is exchanged between systems. Work involves a force acting over a distance—like a piston compressing gas—where energy transfer is directed and ordered.
Heat transfer, conversely, stems from thermal gradients, spreading energy in a more randomized manner. In thermodynamics, these interactions are not interchangeable without cost.
Heat transferred can do work, but never completely. This fundamental asymmetry sets the stage for the second law and introduces the concept of entropy, the measure of energy’s dispersal and unavailability.
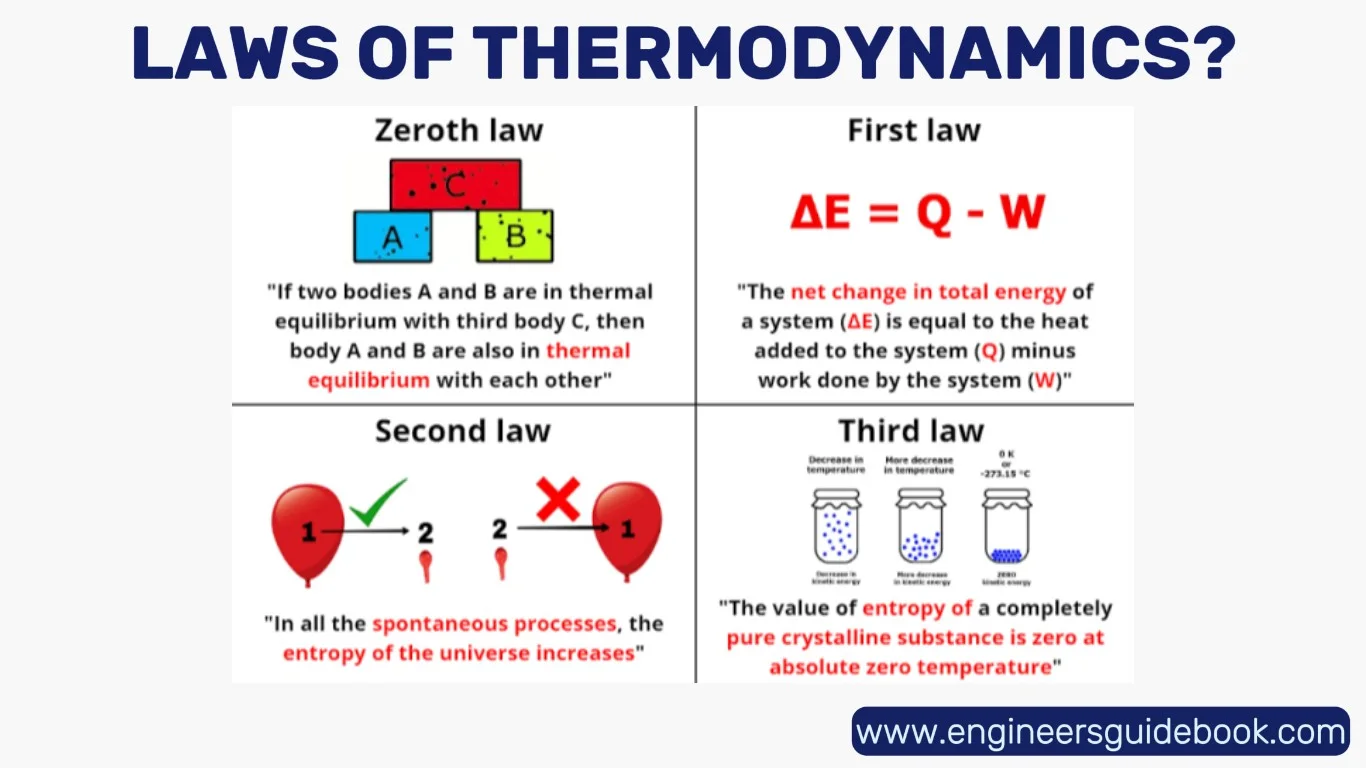
5. The Zeroth Law of Thermodynamics
The Zeroth Law of Thermodynamics, though formulated after the first and second laws, serves as the bedrock for the concept of temperature. It articulates a seemingly simple but profoundly important idea: if two systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other. This law enables the creation of a consistent temperature scale, laying the foundation for thermal measurement and calibration.
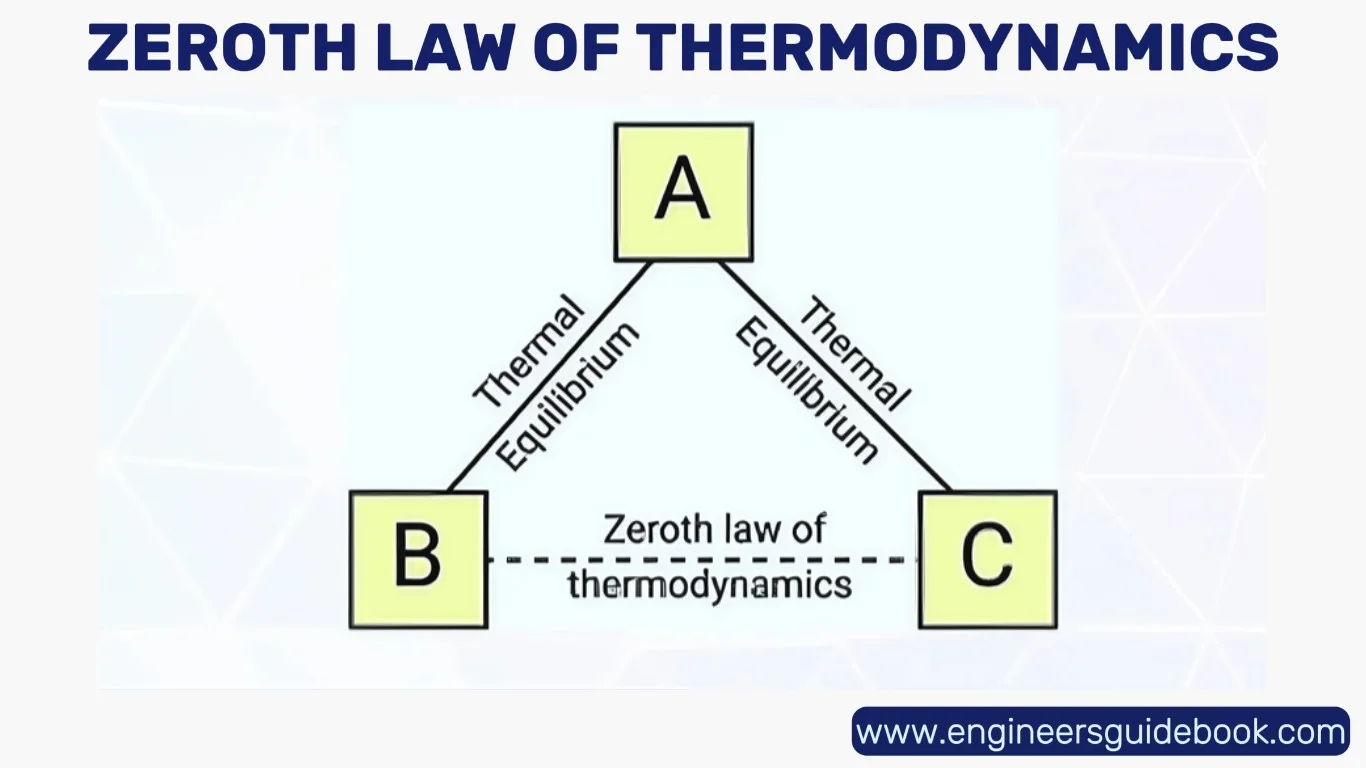
5.1 Establishing Thermal Equilibrium
Thermal equilibrium occurs when two bodies in thermal contact cease to exchange heat, reaching a state of energetic parity.
No heat flows between them, not because they’re isolated, but because their temperatures are equal.
This principle is what allows the comparison of temperatures between objects and systems. In this context, temperature becomes a measurable, transitive property, capable of being quantified and standardized across systems of any scale.
5.2 Real-World Applications
Thermometers exploit the Zeroth Law to function accurately. When a thermometer is placed in contact with an object, it reaches thermal equilibrium with that object. The thermometer’s response often a change in volume of a liquid or resistance in a material—correlates directly to temperature.
This law justifies the use of temperature scales such as Celsius, Fahrenheit, and Kelvin, ensuring that thermal equilibrium measurements are repeatable and meaningful in both daily life and advanced scientific work.
6. The First Law of Thermodynamics
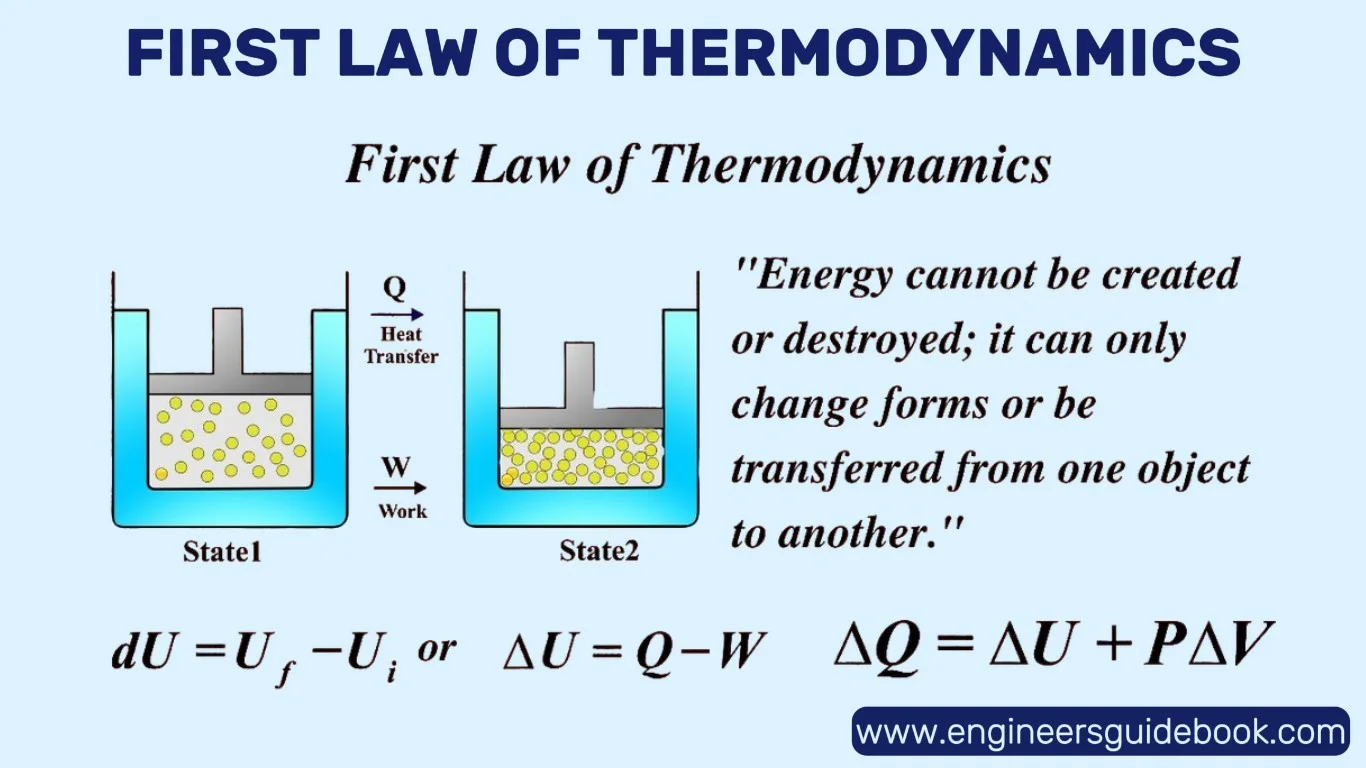
The First Law of Thermodynamics states that energy can neither be created nor destroyed—it can only change form.
This law is the quantitative backbone of thermodynamics, equating heat added to a system with the sum of the increase in internal energy and the work done by the system. It is, in essence, the conservation of energy principle applied to thermal processes.
6.1 Internal Energy and System Boundaries
Internal energy encompasses all the microscopic kinetic and potential energies within a system—vibrations, molecular rotations, atomic bonds, and more.
It’s a function of state, meaning it depends solely on the system’s current condition, not the path taken to arrive there.
Defining system boundaries is essential here; whether the system is open, closed, or isolated determines how energy crosses the border, whether as heat, work, or matter.
6.2 Heat, Work, and the Energy Balance Equation
Mathematically expressed as ΔU = Q − W, the First Law links internal energy change (ΔU) to heat added (Q) and work done (W).
A positive Q increases energy, while positive W (work done by the system) reduces it. This balance governs countless real-world processes—from the expansion of gases to chemical reactions—ensuring that every joule of energy is accounted for.
6.3 Practical Examples in Mechanical and Chemical Systems
In engines, combustion transforms chemical energy into mechanical work and heat, both governed by the First Law. In chemical reactions, energy absorbed or released manifests as enthalpy change, impacting reaction feasibility.
Heat exchangers, steam turbines, and even your own metabolism—each is an embodiment of energy conservation in action, operating within the parameters set by the First Law.
7. The Second Law of Thermodynamics
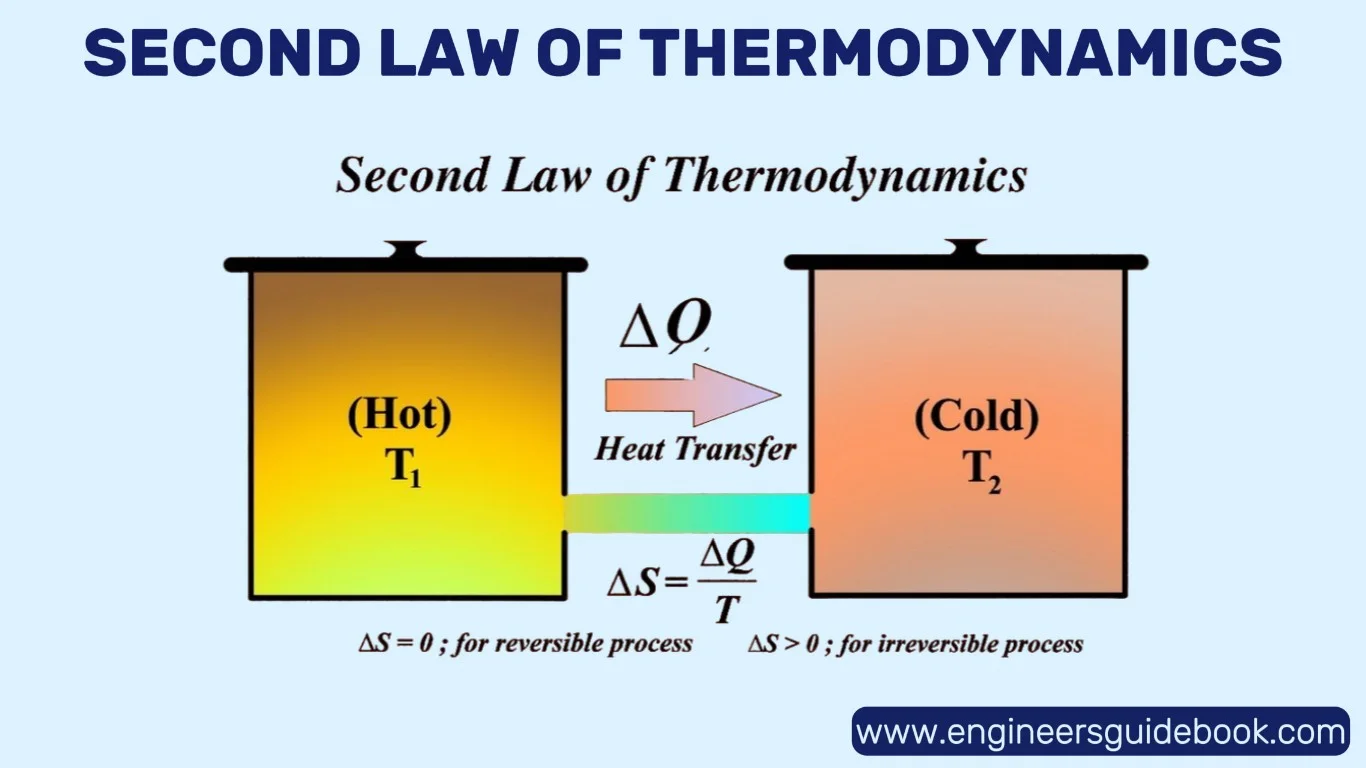
The Second Law introduces a concept that transcends mechanics: entropy. It states that in any isolated system, entropy tends to increase over time.
This is not merely a suggestion of disorder, but a fundamental asymmetry in the nature of time itself. It delineates the possible from the impossible, distinguishing reversible from irreversible processes.
7.1 Entropy: Disorder, Chaos, and the Flow of Time
Entropy, often misunderstood as disorder, is better interpreted as the number of microscopic configurations that correspond to a macroscopic state. A higher entropy state has more possible arrangements, making it statistically more probable.
This law explains why heat flows spontaneously from hot to cold, not the reverse, and why shattered glass doesn’t reassemble. It gives time a direction—an arrow that points from order to chaos, from potential to degradation.
7.2 Real-Life Implications: Refrigerators, Power Plants, and More
The Second Law underpins the design of refrigerators, air conditioners, and heat pumps, all of which move heat against its natural gradient using external work.
In power plants, thermal efficiency is scrutinized in light of entropy generation. Environmental engineering, sustainable energy systems, and even economic theories of productivity echo the principles of this unyielding law.
8. The Third Law of Thermodynamics
The Third Law of Thermodynamics posits that as a system approaches absolute zero temperature, the entropy of a perfect crystal approaches a minimum constant—typically zero.
This law addresses the behavior of matter at extremely low temperatures, where quantum effects begin to dominate and classical physics loses its footing.
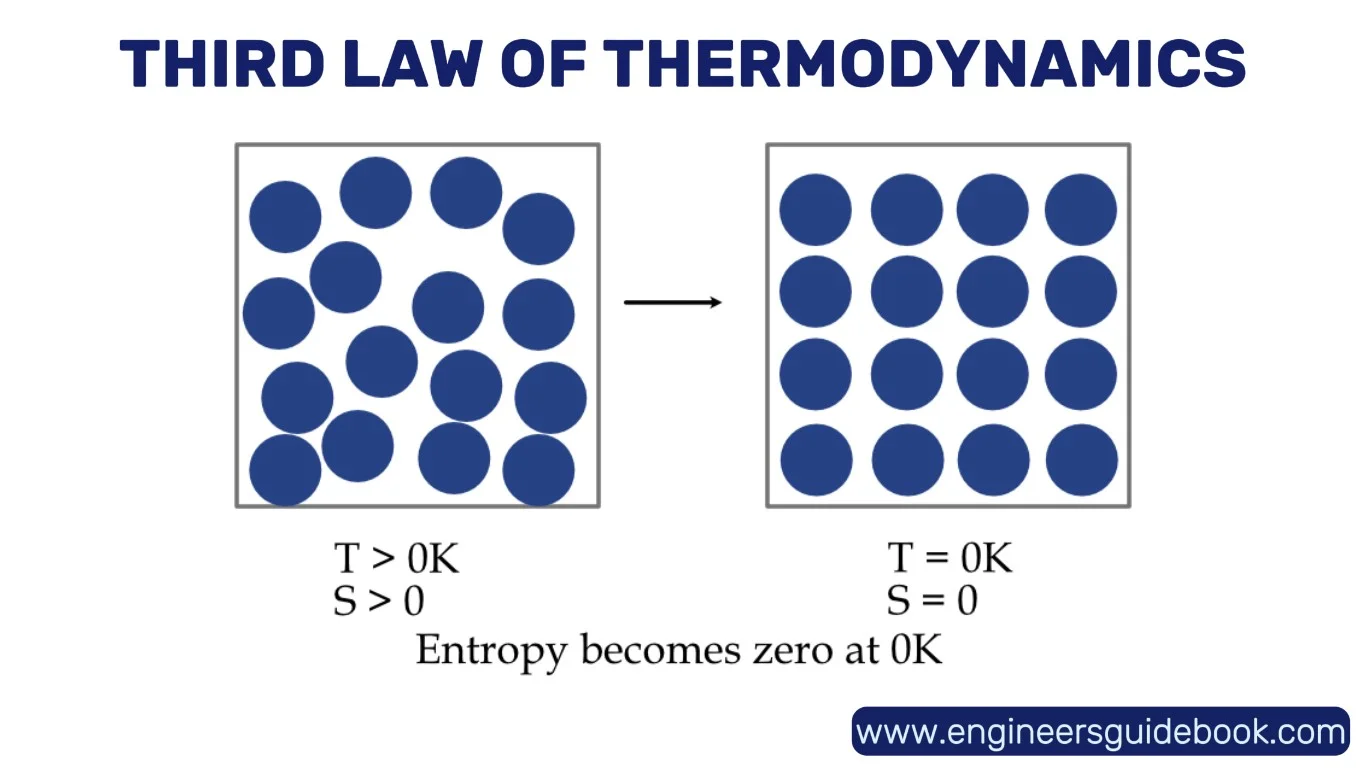
8.1 What Happens as Temperature Approaches Zero?
Approaching absolute zero (−273.15°C or 0 K), thermal motion grinds to a halt. Molecules barely vibrate, and the system’s capacity to do work dwindles to nothing.
Phase transitions slow, chemical reactions become frozen in time, and materials exhibit strange quantum behavior such as superconductivity and superfluidity.
8.2 Practical Constraints in Reaching Absolute Zero
No process, no matter how refined, can reduce a system’s temperature to absolute zero in a finite number of steps. This limitation arises from both thermodynamic law and quantum mechanics.
Ultra-low temperature laboratories use techniques like adiabatic demagnetization and laser cooling to inch ever closer to this theoretical limit, enabling groundbreaking research in quantum computing and particle physics.
Nonetheless, the Third Law ensures that absolute zero remains an unbreakable boundary, a cold horizon that recedes the closer one gets.
9. Thermodynamic Systems and Their Classifications
A thermodynamic system is any defined quantity of matter or region in space under study, separated from its surroundings by a boundary. Understanding the classification of systems is essential to predict and analyze energy exchanges, whether in a laboratory apparatus or a planetary atmosphere.
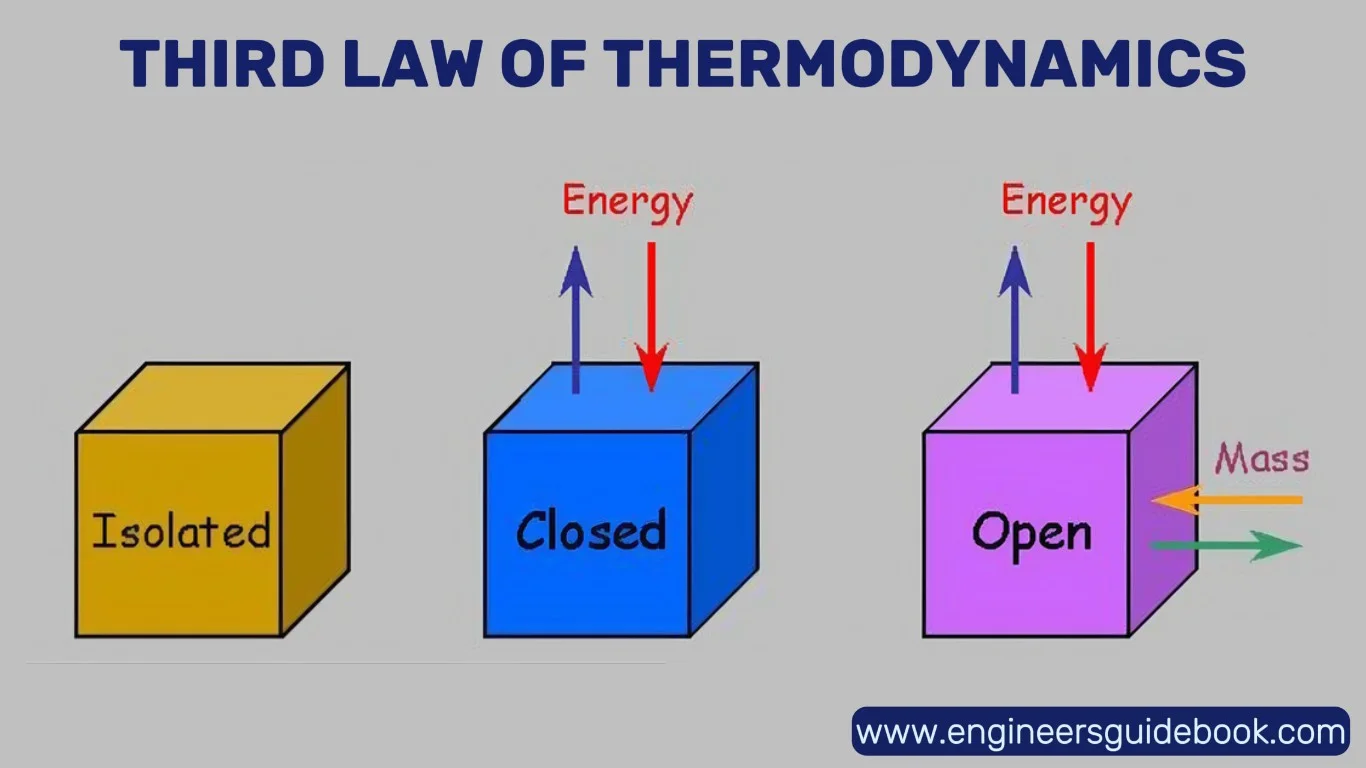
9.1 Open, Closed, and Isolated Systems
Open systems: can exchange both matter and energy with their environment. A boiling pot with no lid is a simple example—steam (mass) escapes and heat (energy) is released.
Closed systems: allow the transfer of energy but not mass. A sealed piston containing gas illustrates this well: heat and work can cross the boundaries, but the gas remains contained.
Isolated systems: theoretical in nature, do not exchange either matter or energy with their surroundings. While a true isolated system doesn’t exist in the real world, approximations—like a vacuum flask—can closely mimic this behavior over short periods.
Each system type governs how we apply thermodynamic laws, impacting everything from engine design to cosmological models.
10. Thermodynamic Processes Explained
Thermodynamic processes describe how a system transitions from one state to another. These transitions are governed by constraints on pressure, volume, temperature, and heat transfer, giving rise to distinct classifications that help in system analysis and prediction.
10.1 Isobaric, Isochoric, Isothermal, and Adiabatic Processes
Isobaric process – Pressure remains constant. Heating a gas in a piston with a freely moving lid exemplifies this; the gas expands as it absorbs heat.
Isochoric process – Volume remains constant. In such a scenario, any added heat increases pressure, not volume—ideal for analyzing rigid containers.
Isothermal process – Temperature remains constant. The internal energy stays fixed, meaning any heat added is wholly converted into work.
diabatic process – No heat is exchanged. The system is insulated, and energy change manifests entirely as work. These processes are rapid, occurring too fast for heat transfer to take place.
Each process traces a unique path on a pressure-volume graph, revealing the nuances of energy transformation.
10.2 Graphical Representation: PV Diagrams and Their Interpretation
PV diagrams (Pressure-Volume diagrams) visually represent thermodynamic processes and cycles. Curves on these graphs embody how pressure and volume change during various transformations.
For example, isobaric processes appear as horizontal lines, while adiabatic and isothermal processes generate curved paths with distinct slopes. The area under any curve on a PV diagram represents work done by or on the system.
11. Thermodynamic Cycles and Their Importance
Thermodynamic cycles are sequences of processes that return a system to its original state while converting energy. These cycles are foundational to all heat engines, providing a structured mechanism to harness heat and turn it into useful work.
11.1 The Carnot Cycle
The Carnot cycle is a theoretical construct composed of two isothermal and two adiabatic processes. It represents the maximum possible efficiency any heat engine can achieve, determined solely by the temperatures of the heat source and sink. While no real engine can match its performance, the Carnot cycle serves as the gold standard, guiding innovations in energy systems.
11.2 Rankine and Otto Cycles
The Rankine cycle powers most thermal power plants, using water and steam to convert heat into mechanical energy. It comprises pumping, boiling, expansion, and condensation steps.
The Otto cycle, the backbone of gasoline engines, consists of isentropic compression and expansion, with constant-volume heat addition and rejection. These cycles are crucial to transportation and energy infrastructure, shaping the efficiency and power of vehicles, turbines, and industrial processes.
11.3 Efficiency Calculations and Limitations
Efficiency is calculated as the ratio of useful work output to energy input. In thermodynamic cycles, this value is always less than 100% due to entropy and irreversibilities.
Factors like friction, heat loss, and incomplete combustion degrade real-world efficiency. Understanding these limitations informs better design, maintenance, and innovation in energy systems.
12. Thermodynamics in Modern Science and Technology
Beyond engines and heat pumps, thermodynamics permeates the cutting edge of research and innovation. It is no longer just about steam and pistons—it is about the architecture of the universe itself.
12.1 Thermodynamics in Chemical Reactions and Biological Systems
Chemical reactions hinge on enthalpy, entropy, and Gibbs free energy—thermodynamic quantities that dictate whether a reaction will proceed spontaneously.
In biological systems, thermodynamics governs enzyme activity, metabolic efficiency, and energy conservation in cellular respiration. Life, at its core, is a thermodynamically driven phenomenon.
12.2 The Role of Thermodynamics in Climate Modeling and Engineering
Climate models rely on thermodynamic equations to simulate the flow of energy through the atmosphere and oceans. Concepts like radiative forcing, heat transfer, and phase change dynamics help predict weather patterns and long-term climate shifts.
In engineering, thermodynamics shapes HVAC systems, sustainable building design, and waste heat recovery—directly impacting energy conservation and carbon reduction.
12.3 Advances in Quantum Thermodynamics
At the frontier lies quantum thermodynamics, where classical laws intersect with quantum uncertainty. Here, particles can tunnel through barriers, exhibit superposition, and follow probabilistic rules. Thermodynamic concepts are being reimagined at the atomic scale, paving the way for innovations in quantum computing, nanoscale engines, and energy-efficient quantum devices.
14. Conclusion
Thermodynamics is more than a set of scientific principles—it is a framework for understanding the flow of energy across the cosmos. From steam engines to stars, from biological cells to black holes, it dictates the possibilities and limitations of transformation.
As technology evolves and human understanding deepens, thermodynamics remains as relevant as ever—anchoring discovery, guiding innovation, and revealing the hidden order behind nature’s seeming chaos.


3 Responses
I don’t even know how I ended up here, but I thought this post was great.
I do not know who you are but definitely you are going to a famous blogger if you aren’t already 😉 Cheers!
Excellent article. enjoy your writings and looking forword to it
Hi, yeah this paragraph is actually fastidious and I have learned lot of things from it about blogging.
thanks.