1. Introduction
The Zeroth Law of Thermodynamics, though less talked about than its numerical successors, serves as the invisible bedrock of thermal science. It articulates a fundamental truth about the thermal interactions of matter—a truth so intuitively accepted that it remained unstated until the necessity of formalism arose.
Ironically, though labeled the “Zeroth” law, it was actually formulated after the First and Second Laws, and assigned its position retroactively to underscore its primacy in logic.
2. Historical Context
In the early 20th century, the need to underpin the concept of temperature with a rigorous scientific basis led to the articulation of what we now call the Zeroth Law.
British physicist Ralph H. Fowler is credited with formalizing the law in the 1930s. Prior to this, temperature was treated as an abstract, albeit measurable, quantity. However, in order to construct a cohesive thermodynamic framework, scientists recognized the necessity of defining thermal equilibrium as a transitive relation.
This retrospective naming reflects the law’s foundational nature. Without it, thermodynamics would lack the scaffolding needed to discuss temperature coherently.
Read More:
Laws of Thermodynamics | A Detailed Guide
3. Zeroth Law of Thermodynamics
Formally, the Zeroth Law of Thermodynamics states:
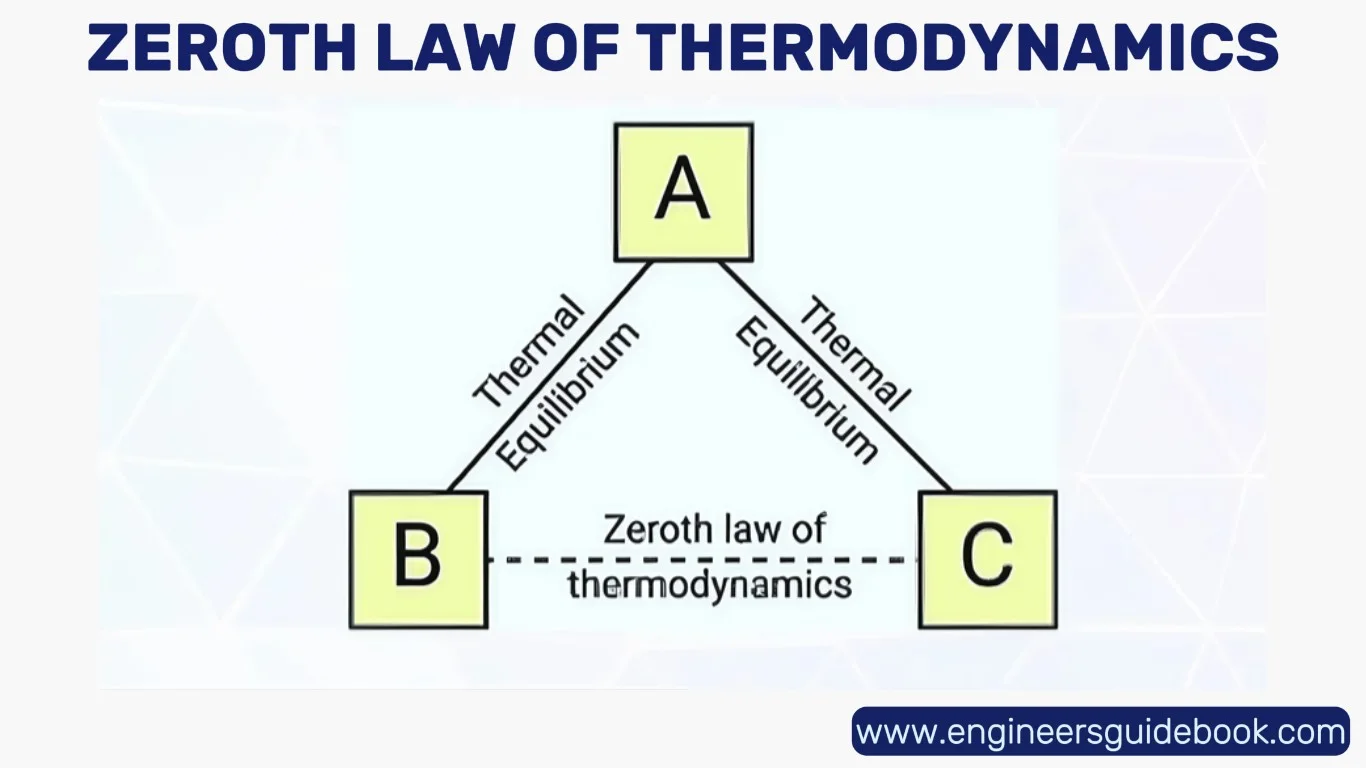
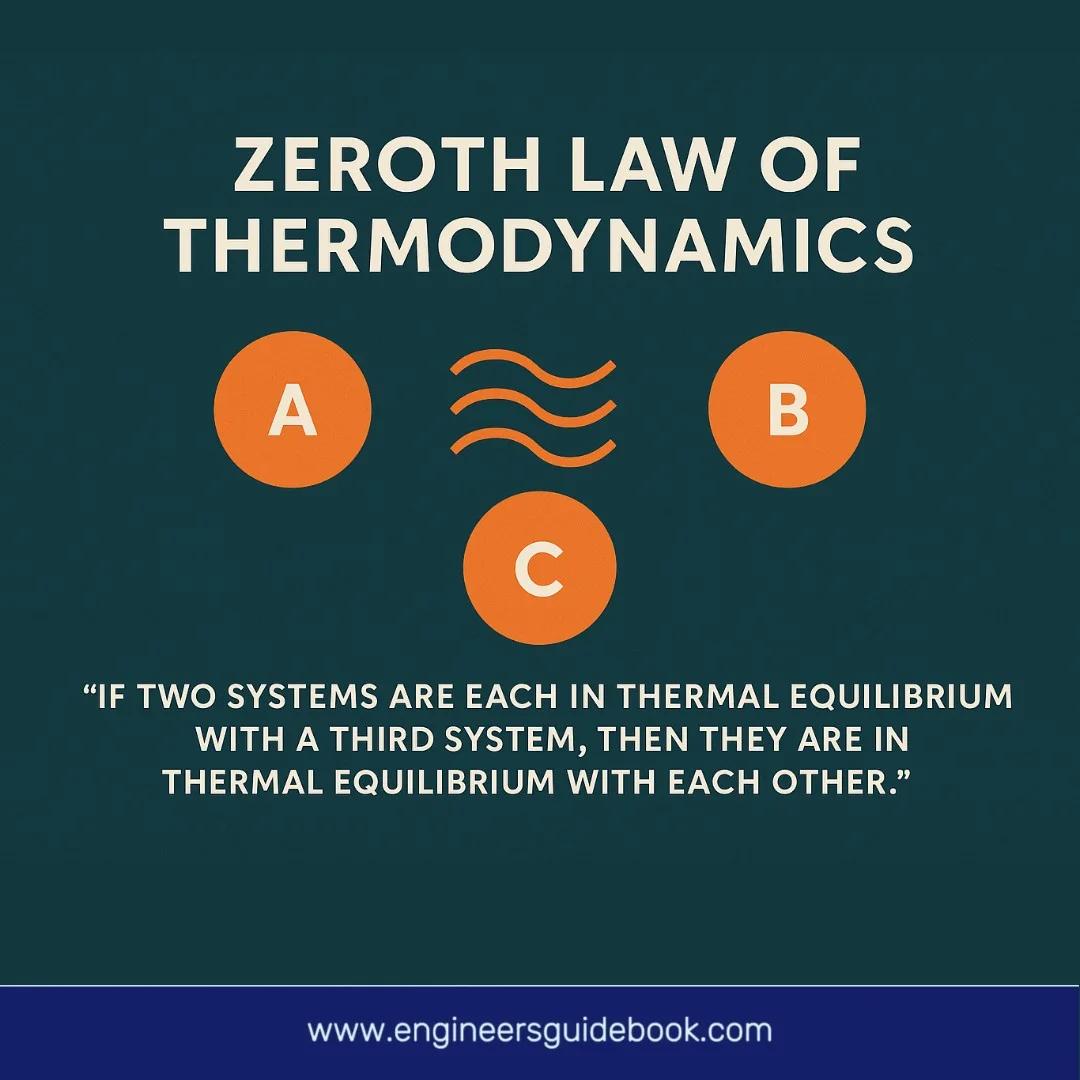
“If two thermodynamic systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other.”
This deceptively simple assertion unlocks profound implications. It defines thermal equilibrium as a transitive relation—a cornerstone concept in physics that underpins the very definition of temperature. In essence, the law tells us that temperature is the property that determines whether or not heat will flow between systems.
4. Thermal Equilibrium and Transitivity
Consider three systems: A, B, and C. If system A is in thermal equilibrium with system B, and system B is in equilibrium with system C, then logically, system A must also be in equilibrium with system C. This transitive nature permits a coherent ordering of systems based on thermal states.
Thermal equilibrium implies that there is no net exchange of energy via heat between systems. It is a state of stasis in the microscopic dance of molecules—a dynamic yet balanced condition where the energetic tendencies cancel out perfectly.
5. The Importance of the Law
While the First Law discusses conservation of energy and the Second Law addresses entropy and spontaneity, the Zeroth Law establishes the groundwork upon which these ideas can operate. Without it, the concept of temperature would remain undefined, and the practice of measuring thermal states would be an exercise in futility.
In fact, all temperature measurement hinges on the Zeroth Law. It provides the logical structure that allows us to say, “this object is hotter than that one,” and to quantify that hotness consistently.
6. Temperature Scales and Thermometers
The practical outcome of the Zeroth Law is the development and calibration of thermometers. When a thermometer is placed in contact with a system and allowed to reach thermal equilibrium, it mirrors the system’s temperature.
Whether using Celsius, Fahrenheit, or Kelvin scales, the thermometer’s reading is valid precisely because of the Zeroth Law. This principle assures us that if our instrument is in thermal equilibrium with multiple systems, all those systems share the same temperature.
The thermometer thus becomes the “third system” referenced in the law. It acts as a thermal mediator, silently enforcing equilibrium across otherwise disconnected entities.
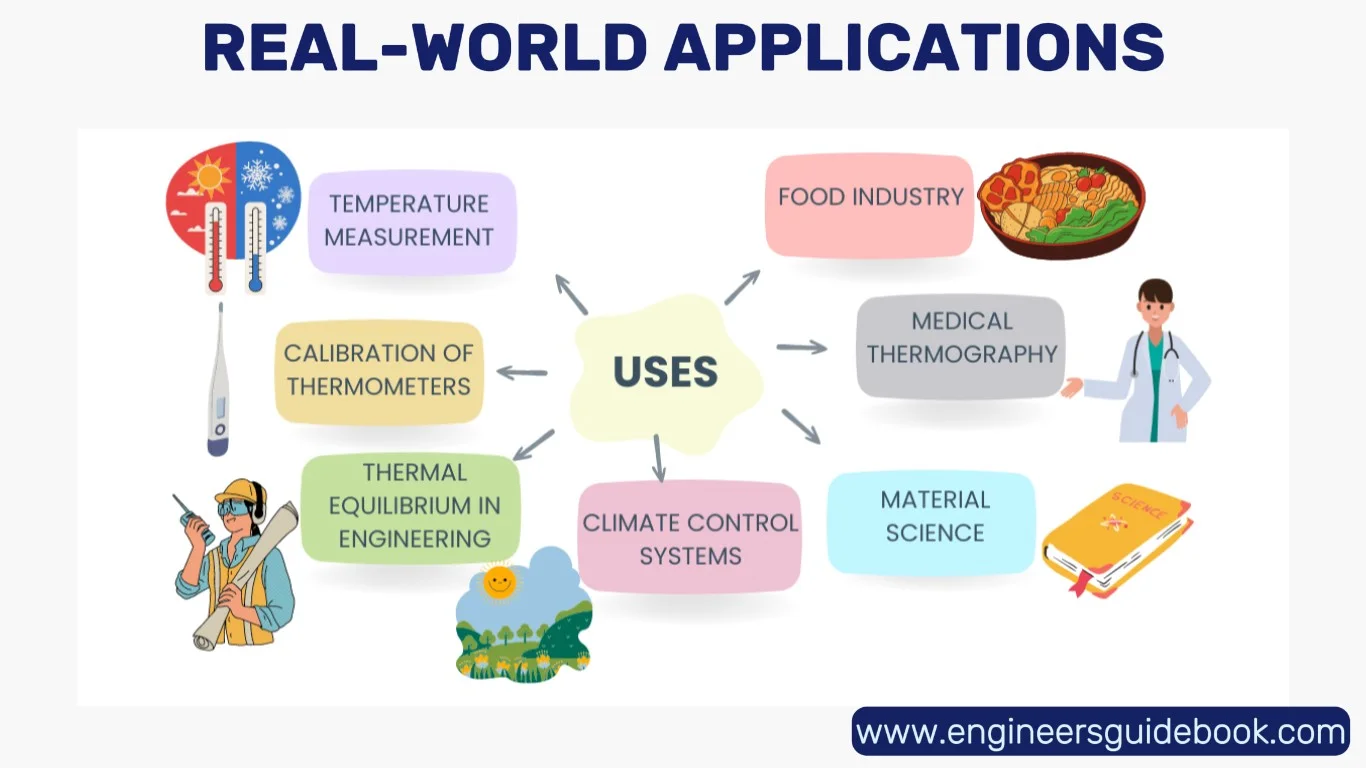
7. Real-World Applications
From industrial furnaces to air conditioning systems, the Zeroth Law finds ubiquitous relevance. In metallurgy, precise temperature control ensures desired crystalline structures. In electronics, thermal management prevents overheating and failure.
Even in medical diagnostics—think of digital thermometers or thermal imaging devices—the principle of thermal equilibrium guides accurate readings.
Materials science leverages the law when testing thermal conductance or behavior under heat stress. The silent assurance that temperatures can be compared reliably undergirds a multitude of scientific and engineering disciplines.
8. Conclusion
The Zeroth Law of Thermodynamics is not flashy, but it is indispensable. Like the quiet sentinel at the gates of scientific rigor, it ensures the consistency of thermal measurements across systems. By establishing the transitive nature of thermal equilibrium, it legitimizes the very concept of temperature—a variable so common, yet so foundational.
In a field often dominated by entropy, engines, and energy conservation, the Zeroth Law remains the serene axiomatic truth: simple, silent, and absolutely essential.



One Response
excellent points altogether, you simply gained a new reader. What would you suggest about your put up that you made a few days in the past? Any certain?