1. Introduction
Enthalpy is a fundamental concept in thermodynamics that governs the principles of energy conservation and transfer. It is a property that helps scientists and engineers understand how energy is absorbed, stored, and released in various physical and chemical processes. Without a clear grasp of enthalpy, it would be challenging to analyze and optimize systems involving heat exchange, chemical reactions, and phase transitions.
1.1 How Enthalpy Relates to Energy and Heat Transfer
Enthalpy represents the total heat content of a system and is intrinsically linked to energy and heat transfer. When a substance undergoes a transformation—such as heating, cooling, or undergoing a phase change—enthalpy quantifies the energy required or released. This concept is essential in designing thermal systems, predicting reaction behaviors, and ensuring energy efficiency in industrial applications.
1.2 Real-World Applications of Enthalpy in Engineering and Science
From power plants to refrigeration systems, enthalpy plays a crucial role in various engineering and scientific fields. It is used in designing heat exchangers, evaluating fuel efficiency in combustion engines, and optimizing industrial chemical reactions. Understanding enthalpy allows engineers to enhance the performance of energy systems and develop sustainable technologies.
2. Definition of Enthalpy
2.1 Formal Definition of Enthalpy in Thermodynamics
Enthalpy is a thermodynamic property defined as the sum of a system’s internal energy and the energy associated with its pressure and volume. It serves as a useful measure of energy transfer in systems that involve heat exchange at constant pressure.
2.2 Mathematical Expression of Enthalpy (H = U + PV)
Enthalpy is mathematically expressed as:
H = U + PV
where:
- H is the enthalpy,
- U is the internal energy,
- P is the pressure,
- V is the volume of the system.
- This equation encapsulates the total energy content of a system, considering both its stored energy and the work required to maintain its volume under pressure.
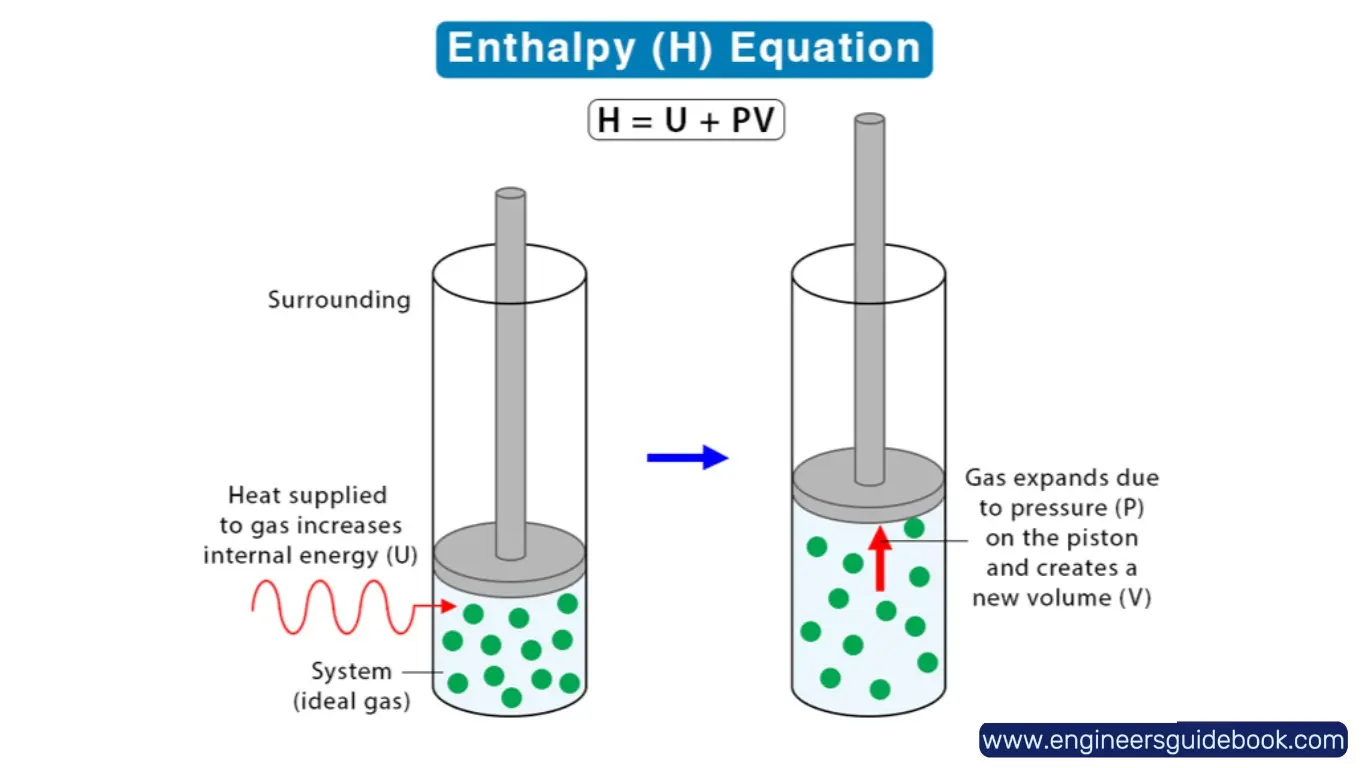
2.3 Breaking Down the Components: Internal Energy (U), Pressure (P), and Volume (V)
- Internal Energy (U): Represents the microscopic kinetic and potential energy of molecules in a system.
- Pressure (P): The force exerted per unit area within the system.
- Volume (V): The space occupied by the system. Understanding these components provides insights into how energy transformations occur in real-world thermodynamic processes.
3. Historical Background of Enthalpy
3.1 Origins of the Concept of Enthalpy
The concept of enthalpy emerged from the broader study of thermodynamics in the 19th century. Scientists sought to quantify heat flow in systems undergoing expansion and compression, leading to the formulation of enthalpy as a state function.
3.2 Contributions of Josiah Willard Gibbs and Heike Kamerlingh Onnes
Josiah Willard Gibbs introduced the idea of thermodynamic potentials, which laid the groundwork for enthalpy. Heike Kamerlingh Onnes further refined thermodynamic properties through his studies on liquefied gases, contributing to the practical application of enthalpy in material science and engineering.
3.3 Evolution of the Enthalpy Concept in Modern Science
Over time, enthalpy has evolved into a critical tool in various scientific and engineering disciplines. Advances in calorimetry, computational modeling, and experimental thermodynamics have expanded its applicability, making it indispensable in energy management and industrial processes.
4. The First Law of Thermodynamics and Enthalpy
4.1 Conservation of Energy Principle and Its Relation to Enthalpy
The First Law of Thermodynamics states that energy cannot be created or destroyed, only transferred or converted. Enthalpy embodies this principle by accounting for heat exchange and work done in a thermodynamic system.
4.2 Enthalpy as a State Function
Enthalpy depends only on the current state of a system, not on the path taken to reach that state. This characteristic makes it a convenient variable for analyzing energy changes in thermodynamic processes.
4.3 Work and Heat Interactions in an Open System
In open systems, where matter and energy can cross boundaries, enthalpy helps quantify the balance between heat transfer and work done by the system, playing a crucial role in applications such as steam turbines and HVAC systems.
5. Enthalpy and Heat Transfer
5.1 The Role of Enthalpy in Heat Absorption and Release
Enthalpy measures the heat absorbed or released during physical and chemical transformations. It is essential for understanding phase changes, chemical reactions, and heat exchanger design.
5.2 Specific Enthalpy: Understanding Enthalpy Per Unit Mass
Specific enthalpy h is defined as enthalpy per unit mass:
h = H / m
Where m is the mass of the substance? This property is particularly useful in engineering applications such as steam tables and refrigeration cycles.
Enthalpy variations occur in isobaric, isochoric, isothermal, and adiabatic processes. Understanding these changes is crucial for designing thermodynamic systems, such as gas turbines and refrigeration units.
6. Units and Measurement of Enthalpy
6.1 Common Units of Enthalpy: Joules, Calories, and BTUs
Enthalpy is typically measured in joules (J), calories (cal), and British Thermal Units (BTUs), depending on the context and industry standards.
6.2 Measuring Enthalpy Changes in Laboratory and Industrial Settings
Calorimetry is commonly used to measure enthalpy changes in controlled environments. Industrial settings rely on process instrumentation to monitor enthalpy variations in real-time.
Devices such as bomb calorimeters, differential scanning calorimeters, and enthalpy meters are employed to assess enthalpy variations accurately. Advanced computational techniques also aid in predictive modeling of enthalpy-driven processes.
7. Enthalpy Change: ΔH and Its Significance
7.1 What is Enthalpy Change and Why is It Important?
Enthalpy change (ΔH) represents the difference in enthalpy between the initial and final states of a system. It helps determine the heat flow in chemical and physical transformations.
7.2 Exothermic vs. Endothermic Processes: Understanding Heat Flow
- Exothermic processes release heat (ΔH < 0), such as combustion and condensation.
- Endothermic processes absorb heat (ΔH > 0), including melting and vaporization. Understanding these processes is essential for energy-efficient system design and industrial operations.
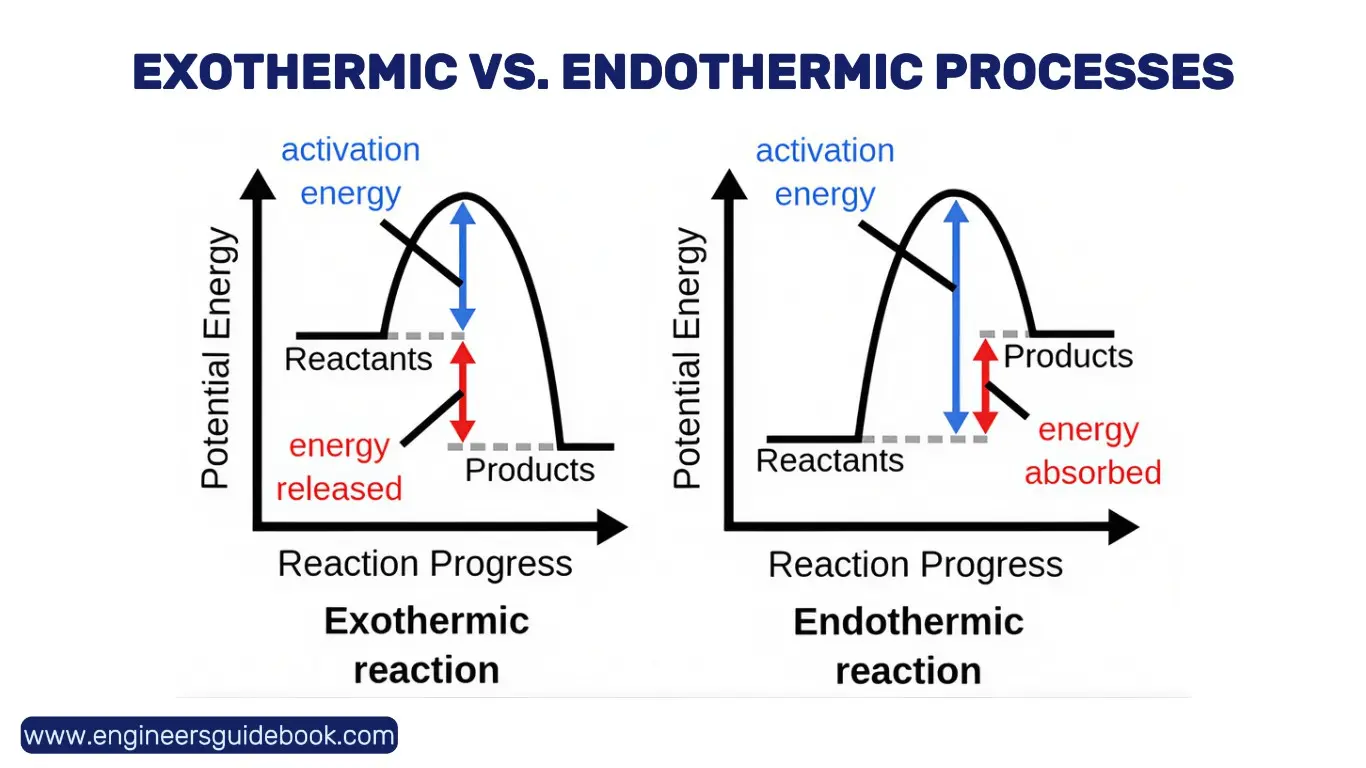
7.3 The Role of Enthalpy Change in Predicting Reaction Feasibility
ΔH is a key parameter in assessing reaction spontaneity. In combination with entropy and Gibbs free energy, it aids in determining whether a reaction will proceed under given conditions, making it a vital tool in chemical engineering and material science.
8. Standard Enthalpy and Reference Conditions
8.1 Definition of Standard Enthalpy Change (ΔH°)
Standard enthalpy change (ΔH°) refers to the enthalpy change associated with a process when all reactants and products are in their standard states. It is a fundamental thermodynamic quantity used to compare different reactions under uniform conditions. The standard state is typically defined at a pressure of 1 bar and a specified temperature, usually 298.15 K (25°C). ΔH° values are extensively used in chemical thermodynamics to determine the heat exchange during reactions.
8.2 Standard Temperature and Pressure (STP) in Enthalpy Calculations
Standard Temperature and Pressure (STP) provides a reference framework for measuring and comparing enthalpy changes. STP is conventionally set at 273.15 K (0°C) and 1 atm pressure. However, thermodynamic standard conditions often differ, with 298.15 K (25°C) and 1 bar being more commonly used. By using STP, enthalpy values can be consistently applied in calculations, enabling precise thermodynamic predictions.
8.3 The Role of Standard Enthalpy in Thermochemical Data
Standard enthalpy values serve as essential reference points in thermochemistry. They enable chemists and engineers to predict reaction spontaneity, determine energy efficiency, and design industrial processes. Thermochemical tables list standard enthalpies of formation, combustion, and phase transitions, facilitating accurate calculations for energy balances in engineering and scientific applications.
9. Enthalpy of Different Processes
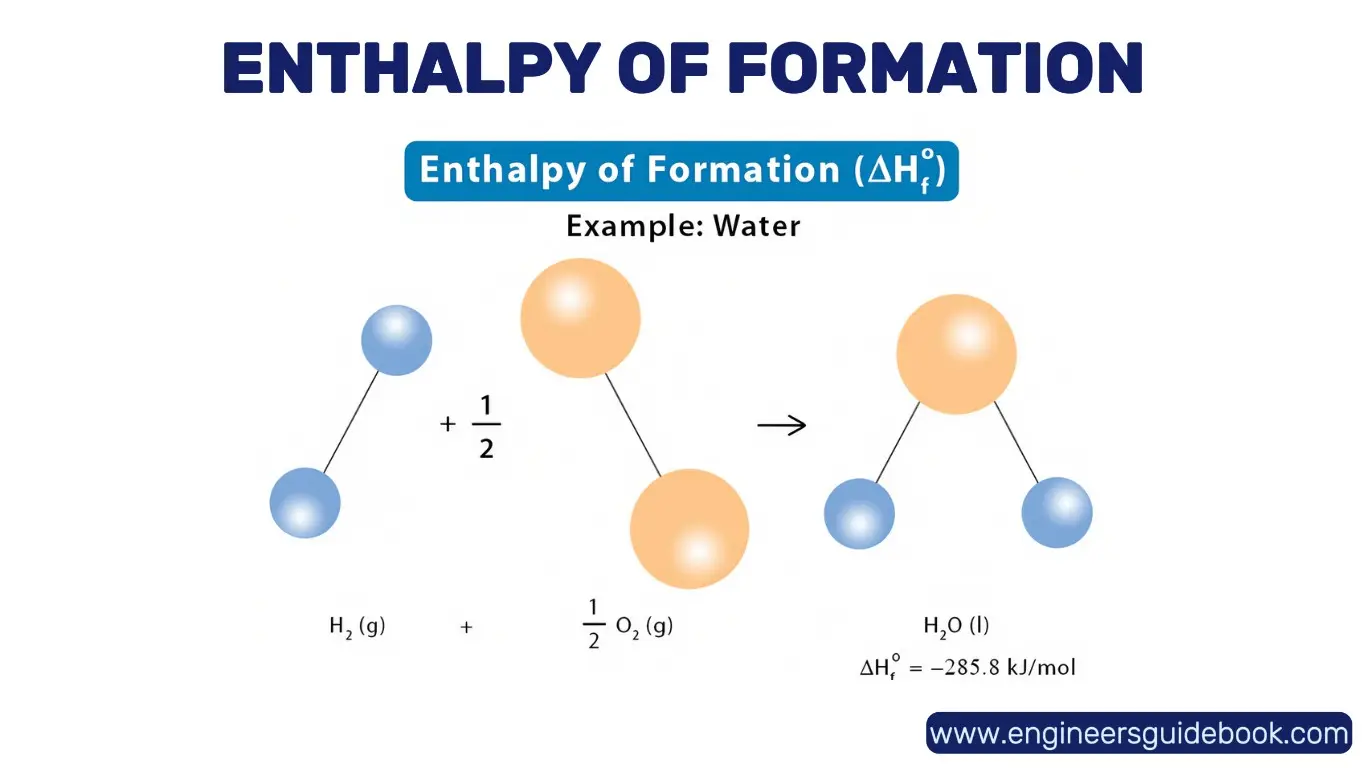
9.1 Enthalpy of Formation: Creating Compounds from Elements
The enthalpy of formation (ΔH_f°) is the enthalpy change when one mole of a compound forms from its constituent elements in their standard states. It provides crucial insights into compound stability and reactivity. A negative ΔH_f° indicates an exothermic and energetically favorable formation, while a positive value suggests an endothermic process.
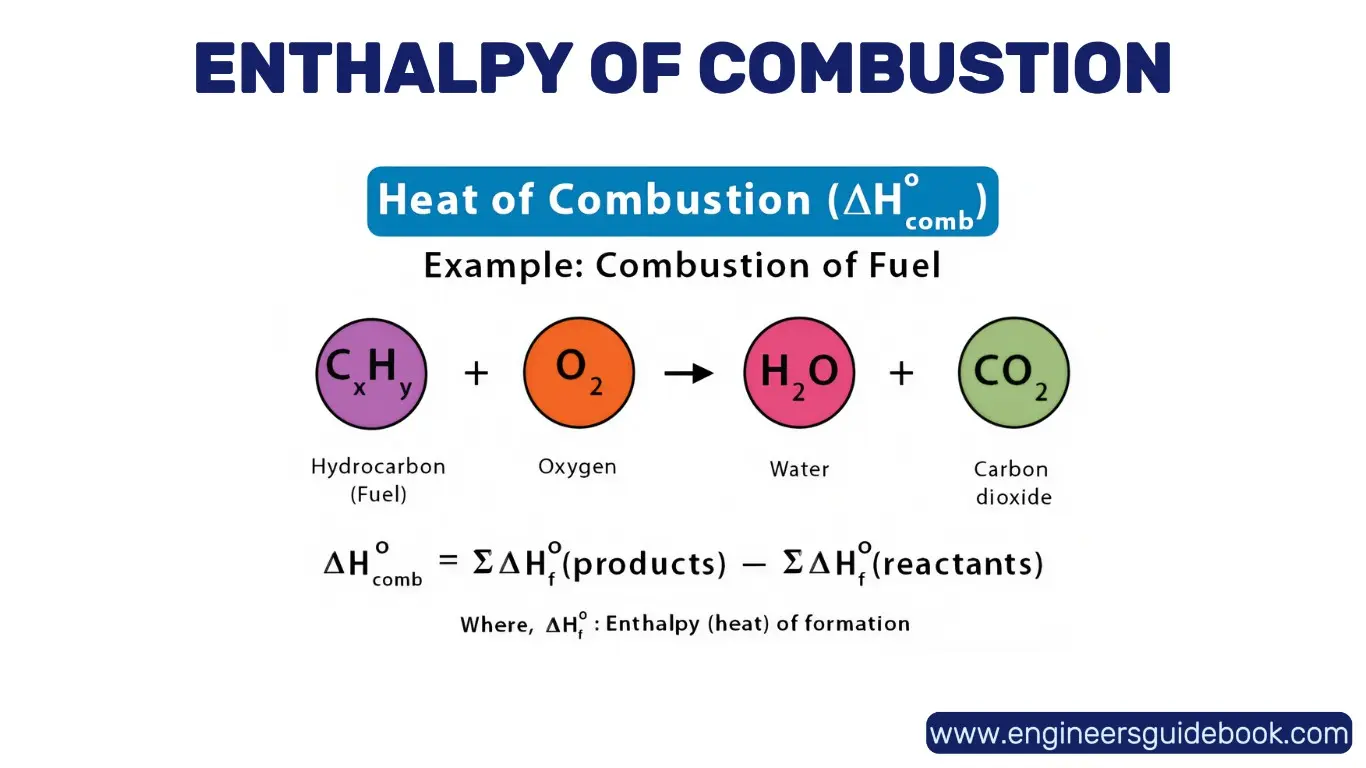
9.2 Enthalpy of Combustion: Heat Released During Burning
The enthalpy of combustion (ΔH_c°) quantifies the heat released when one mole of a substance undergoes complete combustion with oxygen under standard conditions. It is a critical parameter in energy production, particularly in fossil fuel and biofuel combustion analysis. High ΔH_c° values indicate substantial energy output, essential for power generation and industrial heating.
9.3 Enthalpy of Vaporization: Phase Transition from Liquid to Gas
The enthalpy of vaporization (ΔH_vap) is the energy required to convert a unit mass or mole of liquid into vapor at constant pressure and temperature. This property is fundamental in distillation, refrigeration, and climate science, as it dictates how substances absorb or release heat during phase transitions.
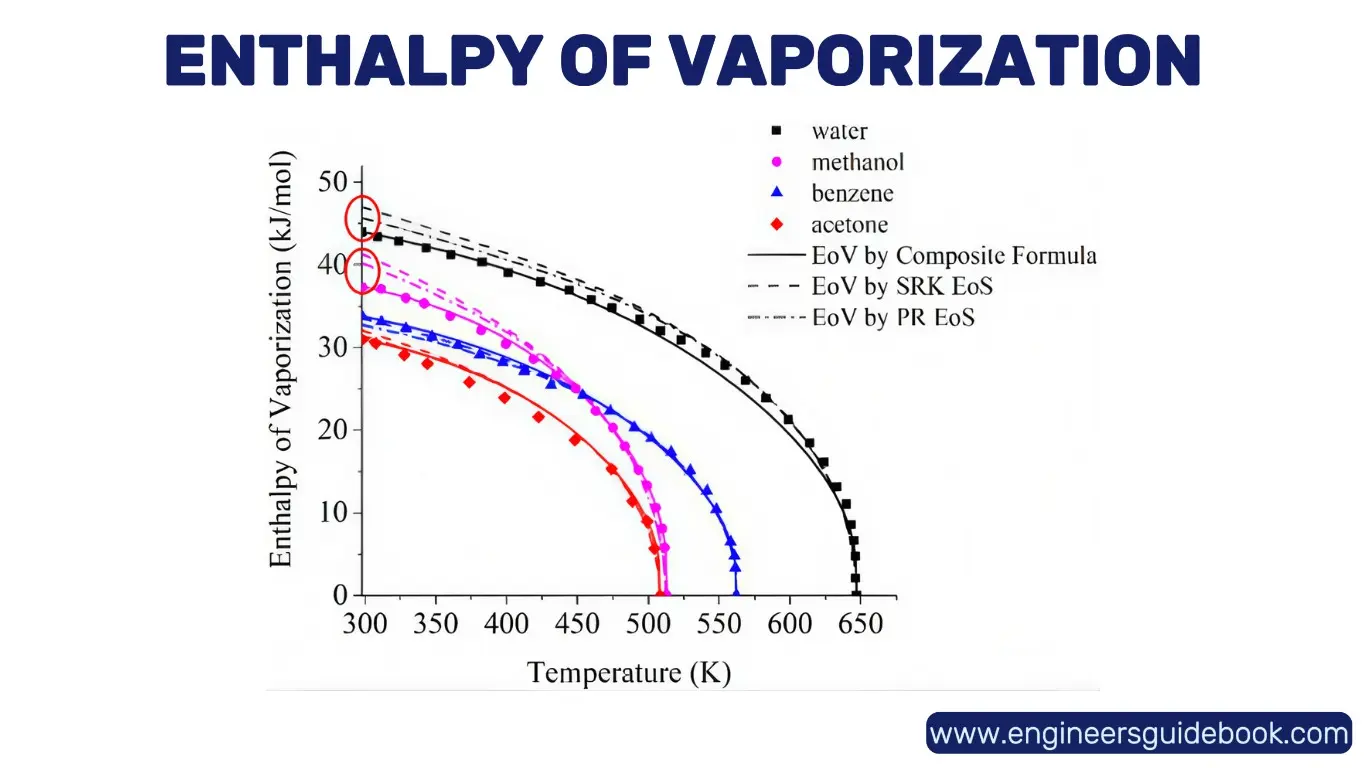
9.4 Enthalpy of Fusion: Melting and Freezing Processes
The enthalpy of fusion (ΔH_fus) represents the heat required to convert a solid into a liquid at constant pressure. It is an essential thermodynamic property in material science and metallurgy. Understanding ΔH_fus allows for precise control of processes like casting, welding, and cryogenic applications.
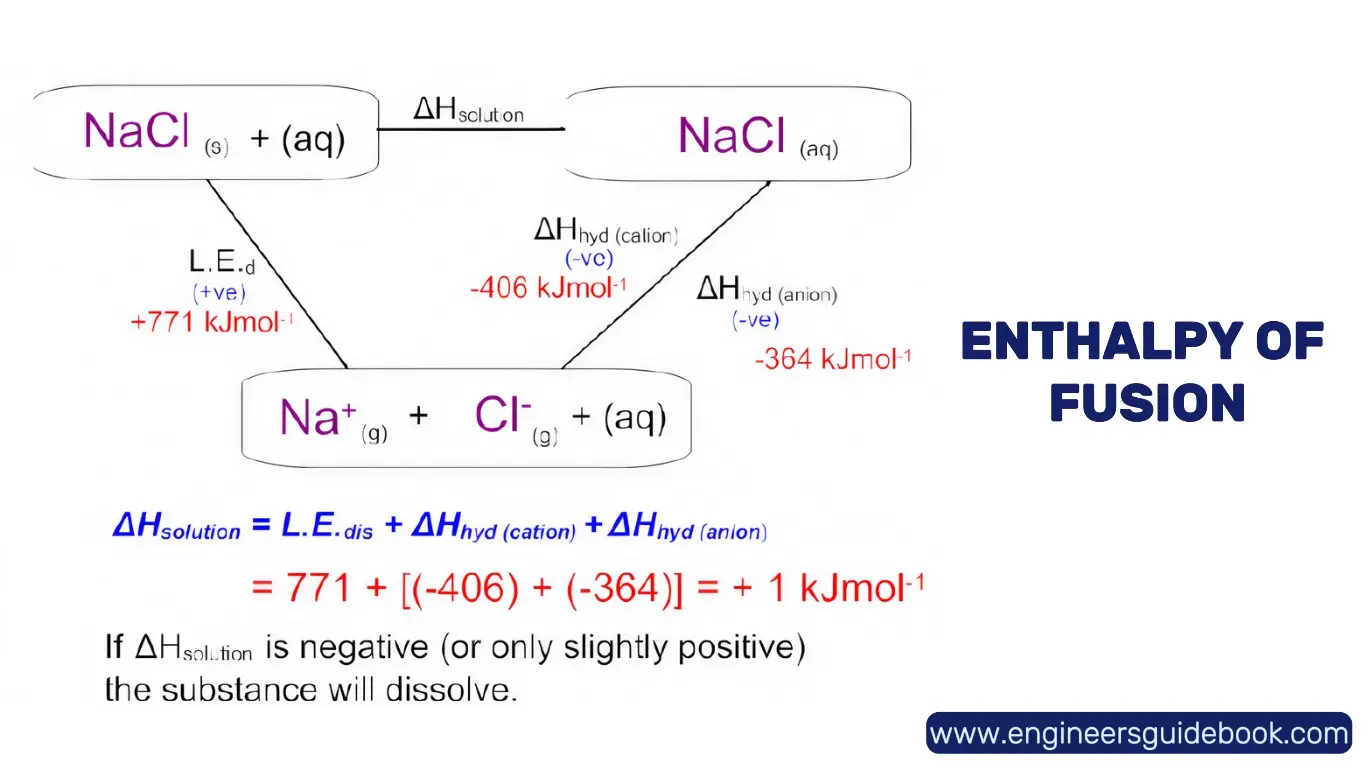
9.5 Enthalpy of Solution: Dissolving Substances in Solvents
The enthalpy of solution (ΔH_sol) measures the heat change when a solute dissolves in a solvent. Depending on the solute-solvent interactions, dissolution can be exothermic or endothermic. This parameter plays a crucial role in chemical manufacturing, pharmaceuticals, and environmental chemistry.
10. Hess’s Law and Enthalpy Calculations
10.1 Understanding Hess’s Law: The Path Independence of Enthalpy
Hess’s Law states that the total enthalpy change of a reaction is independent of the reaction pathway and depends only on the initial and final states. This principle allows complex reactions to be analyzed by summing the enthalpy changes of intermediate steps.
10.2 How to Apply Hess’s Law in Thermodynamic Calculations
Hess’s Law enables enthalpy calculations for reactions that are difficult to measure directly. By breaking down reactions into known enthalpy changes, chemists can determine unknown enthalpy values using standard enthalpy data from thermochemical tables.
10.3 Practical Examples and Problem-Solving Strategies
Practical applications of Hess’s Law include determining fuel combustion efficiency, calculating reaction enthalpies in industrial processes, and designing energy-efficient chemical reactions. By applying stepwise calculations, engineers can optimize reaction conditions and minimize energy losses.
11. Enthalpy in Chemical Reactions
11.1 How Enthalpy Dictates Chemical Reaction Energetics
Enthalpy changes govern reaction spontaneity, determining whether a reaction absorbs or releases heat. Exothermic reactions release energy (negative ΔH), while endothermic reactions absorb energy (positive ΔH), influencing reaction feasibility and industrial applications.
11.2 The Relationship Between Enthalpy and Bond Energy
Breaking and forming chemical bonds involve enthalpy changes. Bond dissociation requires energy input (endothermic), while bond formation releases energy (exothermic). Understanding bond enthalpies allows prediction of reaction energetics and stability.
11.3 Enthalpy Diagrams: Visualizing Energy Changes in Reactions
Enthalpy diagrams graphically represent energy changes in reactions. Reactants and products are plotted, illustrating whether a reaction is exothermic or endothermic. These diagrams aid in conceptualizing reaction mechanisms and energy profiles.
12. Enthalpy and Phase Transitions
12.1 The Role of Enthalpy in Melting, Boiling, and Sublimation
Phase transitions involve enthalpy changes as substances move between solid, liquid, and gaseous states. Each transition requires or releases latent heat, affecting industrial applications such as material processing and cryogenics.
12.2 Latent Heat and Its Connection to Enthalpy Changes
Latent heat refers to the heat absorbed or released during a phase transition without a temperature change. It plays a vital role in climate science, meteorology, and industrial heat transfer processes.
12.3 Enthalpy in Refrigeration and Air Conditioning Systems
Refrigeration cycles rely on enthalpy changes during compression and expansion of refrigerants. Understanding enthalpy variations ensures efficient cooling in HVAC systems, optimizing energy consumption and performance.
13. Enthalpy in Engineering Applications
13.1 Enthalpy in Steam and Power Plants
Steam power generation depends on enthalpy changes in water and steam cycles. High-pressure steam drives turbines, converting thermal energy into mechanical work, making enthalpy a key factor in thermodynamic efficiency.
13.2 The Role of Enthalpy in HVAC and Energy Systems
HVAC systems regulate temperature by manipulating enthalpy changes in refrigerants. Understanding these principles improves energy efficiency and enhances indoor climate control.
13.3 Enthalpy in Aerospace and Jet Engine Design
Jet engines utilize enthalpy variations in high-temperature combustion and exhaust expansion. Engineers optimize enthalpy efficiency to enhance thrust and fuel economy in aviation technology.
14. Enthalpy and the Environment
Atmospheric enthalpy influences weather patterns, air movement, and climate dynamics. Understanding enthalpy flux aids in predicting storms and global temperature variations.
Renewable energy sources, such as geothermal and solar power, rely on enthalpy changes to convert heat into usable energy. Maximizing enthalpy efficiency enhances sustainability.
Storm formation and intensity are linked to enthalpy variations in the atmosphere. Studying these changes helps meteorologists forecast extreme weather events and develop climate models.
15. The Relationship Between Enthalpy and Entropy
15.1 How Enthalpy and Entropy Work Together in Thermodynamics
Thermodynamics revolves around two fundamental properties: enthalpy and entropy. Enthalpy (H) represents the total heat content of a system, reflecting the energy required to create the system and the energy necessary to maintain its pressure and volume. Entropy (S), on the other hand, signifies the degree of disorder or randomness within a system. These two properties are inextricably linked, governing the feasibility and direction of chemical and physical processes.
A reaction’s spontaneity is not determined solely by enthalpy but by the interplay between enthalpy and entropy. While exothermic reactions (ΔH < 0) often lead to spontaneity due to heat release, an increase in entropy (ΔS > 0) can drive endothermic reactions forward. This dynamic equilibrium between heat exchange and disorder underpins the core principles of thermodynamics.
15.2 The Connection Between Enthalpy, Entropy, and Gibbs Free Energy
Gibbs free energy (G) unifies enthalpy and entropy into a single thermodynamic function, providing a comprehensive criterion for spontaneity. Defined by the equation:
ΔG = ΔH – TΔS
where ΔG is the change in Gibbs free energy, ΔH is the enthalpy change, T is the absolute temperature, ΔS is entropy change. This equation dictates that a process is spontaneous when ΔG < 0 , non-spontaneous when ΔG > 0 , and at equilibrium when ΔG = 0 .
At high temperatures, entropy plays a dominant role, potentially driving an otherwise endothermic reaction forward. Conversely, at lower temperatures, enthalpy often dictates the reaction pathway. Understanding this relationship is crucial for fields such as chemical engineering, where reaction conditions must be carefully optimized.
15.3 The Role of Enthalpy in Spontaneous vs. Non-Spontaneous Processes
The classification of processes as spontaneous or non-spontaneous depends on both enthalpy and entropy contributions. Exothermic reactions, such as combustion, typically proceed spontaneously as they release energy, reducing system enthalpy. However, some reactions, like the melting of ice at room temperature, are endothermic yet spontaneous due to a significant increase in entropy.
A non-spontaneous process requires external energy input to proceed. For instance, the decomposition of calcium carbonate (CaCO₃ → CaO + CO₂) is endothermic and necessitates high temperatures to drive the reaction. By analyzing enthalpy and entropy together, engineers and scientists can predict reaction feasibility and design energy-efficient processes.
16. Limitations and Assumptions in Enthalpy Calculations
16.1 Common Assumptions in Enthalpy Calculations
To simplify enthalpy calculations, thermodynamic models often rely on specific assumptions. These include:
- Constant Pressure: Enthalpy calculations assume processes occur at constant pressure, which may not always be valid in real-world applications.
- Ideal Gas Behavior: Many calculations consider gases as ideal, ignoring intermolecular interactions that affect enthalpy values.
- Negligible Work Other Than PV Work: Thermodynamic models often exclude work done by electrical, magnetic, or gravitational forces, focusing only on pressure-volume work.
- Reference States: Standard enthalpy values are based on defined reference conditions (298.15 K, 1 atm), which may differ from actual operational environments.
16.2 When and Why Enthalpy Alone is Not Enough to Describe a System
While enthalpy quantifies heat exchange, it does not account for system disorder or feasibility. For instance, an exothermic reaction might still be non-spontaneous if it significantly reduces entropy. This limitation underscores the necessity of incorporating entropy and Gibbs free energy into comprehensive thermodynamic analyses.
In high-energy applications such as combustion engines, relying solely on enthalpy may lead to misleading conclusions. Additional parameters, including entropy, temperature variations, and reaction kinetics, must be considered for accurate modeling and optimization.
16.3 The Role of Ideal vs. Real Gases in Enthalpy Calculations
Real gases deviate from ideal behavior due to intermolecular forces and finite molecular volumes. These deviations become significant at high pressures and low temperatures, where ideal gas assumptions break down. Corrections, such as those provided by the van der Waals equation, refine enthalpy calculations to reflect actual gas behavior.
17. Advanced Topics in Enthalpy
17.1 Enthalpy in Quantum and Statistical Mechanics
Quantum mechanics offers a microscopic perspective on enthalpy by analyzing molecular energy levels and transitions. Statistical mechanics further refines enthalpy calculations by considering the distribution of molecular states in thermodynamic systems.
17.2 The Role of Enthalpy in High-Pressure and Extreme Temperature Conditions
Enthalpy changes dramatically under extreme conditions, such as those found in deep-sea environments or stellar interiors. High-pressure systems exhibit phase transitions and altered heat capacities, necessitating specialized thermodynamic models.
17.3 Computational Methods for Estimating Enthalpy in Complex Systems
Modern computational techniques, including density functional theory (DFT) and molecular dynamics simulations, allow precise enthalpy estimations for complex molecules and reaction networks. These methods are crucial in fields like materials science and pharmaceutical engineering.
18. Practical Problems and Case Studies
18.1 Solving Real-World Enthalpy Problems Step by Step
Stepwise methodologies for enthalpy calculations enhance precision in engineering applications. These include:
- Determining standard enthalpy changes using Hess’s Law.
- Applying bond enthalpy data for reaction heat estimations.
- Utilizing calorimetry measurements to assess heat transfer.
18.2 Case Study: Enthalpy Changes in Industrial Chemical Reactions
Industries leverage enthalpy calculations to optimize energy efficiency. In ammonia synthesis (Haber process), enthalpy considerations dictate temperature and pressure settings for maximum yield and minimal energy consumption.
18.3 Case Study: Enthalpy Analysis in Power Generation Systems
Power plants rely on enthalpy assessments to enhance thermal efficiency. Steam turbines in Rankine cycles optimize enthalpy differentials to maximize electricity output while minimizing energy waste.
19. FAQ’S
19.1 What is the Difference Between Enthalpy and Internal Energy?
Enthalpy includes internal energy and the energy required to maintain system pressure, making it more applicable to constant-pressure processes.
19.2 How is Enthalpy Used in Everyday Life?
From cooking to climate control, enthalpy governs heat exchange in daily activities, impacting refrigeration, combustion, and even metabolic processes.
19.3 Can Enthalpy Be Negative? Understanding the Significance of ΔH
A negative enthalpy change (exothermic) indicates energy release, while a positive change (endothermic) signifies energy absorption. These values dictate reaction feasibility and practical applications.
20. Conclusion
Enthalpy is a cornerstone of thermodynamics, influencing energy transfer, reaction spontaneity, and industrial efficiency.
Mastery of enthalpy concepts empowers engineers to design energy-efficient systems, optimize chemical processes, and advance scientific research.
Ongoing advancements in computational modeling, nanotechnology, and high-pressure thermodynamics continue to refine our understanding of enthalpy, unlocking new technological frontiers.



3 Responses
This post is great and informational. Thank You for writing this post
Its such as you read my mind! You appear to grasp a lot approximately this,
like you wrote the ebook in it or something. I think that you simply can do with some % to pressure
the message house a bit, but instead of that, that is great blog.
An excellent read. I’ll certainly be back.
great submit, very informative. I’m wondering why the other experts of
this sector don’t realize this. You should continue your writing.
I’m confident, you’ve a great readers’ base already!