1. Introduction to the First Law of Thermodynamics
In engineering, this law informs the design of machines, engines, and infrastructure by ensuring that input and output energies are accounted for with rigorous precision.
Understanding this law is essential to developing sustainable technologies and energy-efficient systems, as it helps predict how energy behaves in closed and open environments.
1.2 Historical Background
Before thermodynamics matured as a discipline, scientists adhered to the caloric theory, which posited that heat was a fluid-like substance flowing from hot bodies to cold ones.
This notion was later dismantled through pivotal experiments by James Joule, Sadi Carnot, and Rudolf Clausius. These thinkers catalyzed the evolution of energy science, culminating in the formulation of the First Law of Thermodynamics.
The shift from viewing heat as a tangible fluid to understanding it as a form of energy was revolutionary and marked the dawn of a new scientific era.
Read More:
Laws of Thermodynamics | A Detailed Guide
Zeroth Law of Thermodynamics|Definition & Applications
2. What Is the First Law of Thermodynamics?
2.1 The Fundamental Statement of the Law
The First Law of Thermodynamics asserts that
Energy can neither be created nor destroyed; it can only be transformed from one form to another.
In other words, the total energy within an isolated system remains constant. This principle, often termed the Law of Energy Conservation, bridges the gap between the abstract notion of energy and its measurable effects on systems in motion or at rest.

2.2 Exploring the Concept of Internal Energy
Internal energy refers to the microscopic energy contained within a substance, arising from the kinetic and potential energies of its molecules.
This internal reservoir fluctuates as heat and work interact with the system. Unlike mechanical energy, internal energy isn’t directly visible, yet it profoundly influences temperature, pressure, and phase changes.
It is an intrinsic property, changing only when energy crosses the system’s boundaries.
3. Mathematical Expression of the First Law
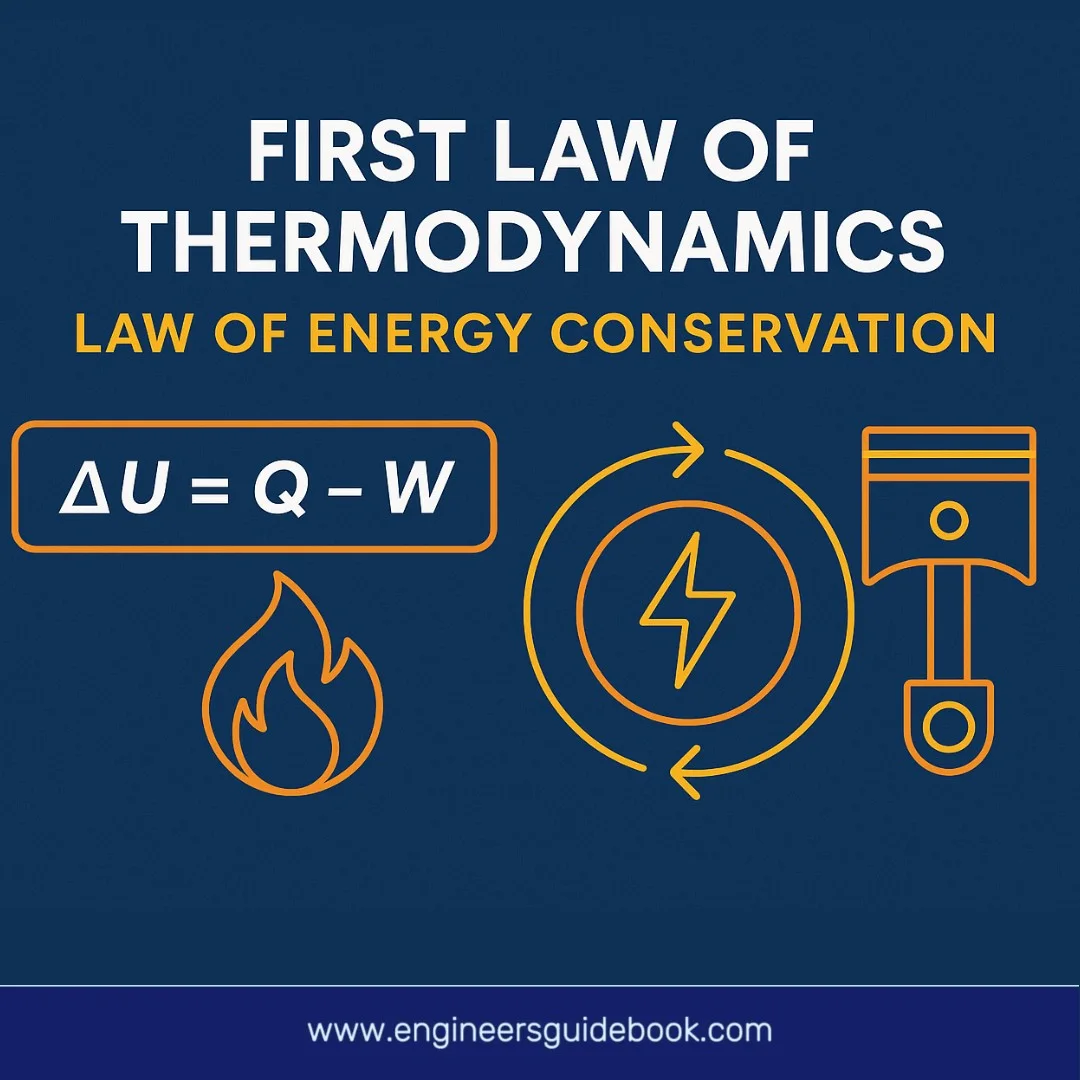
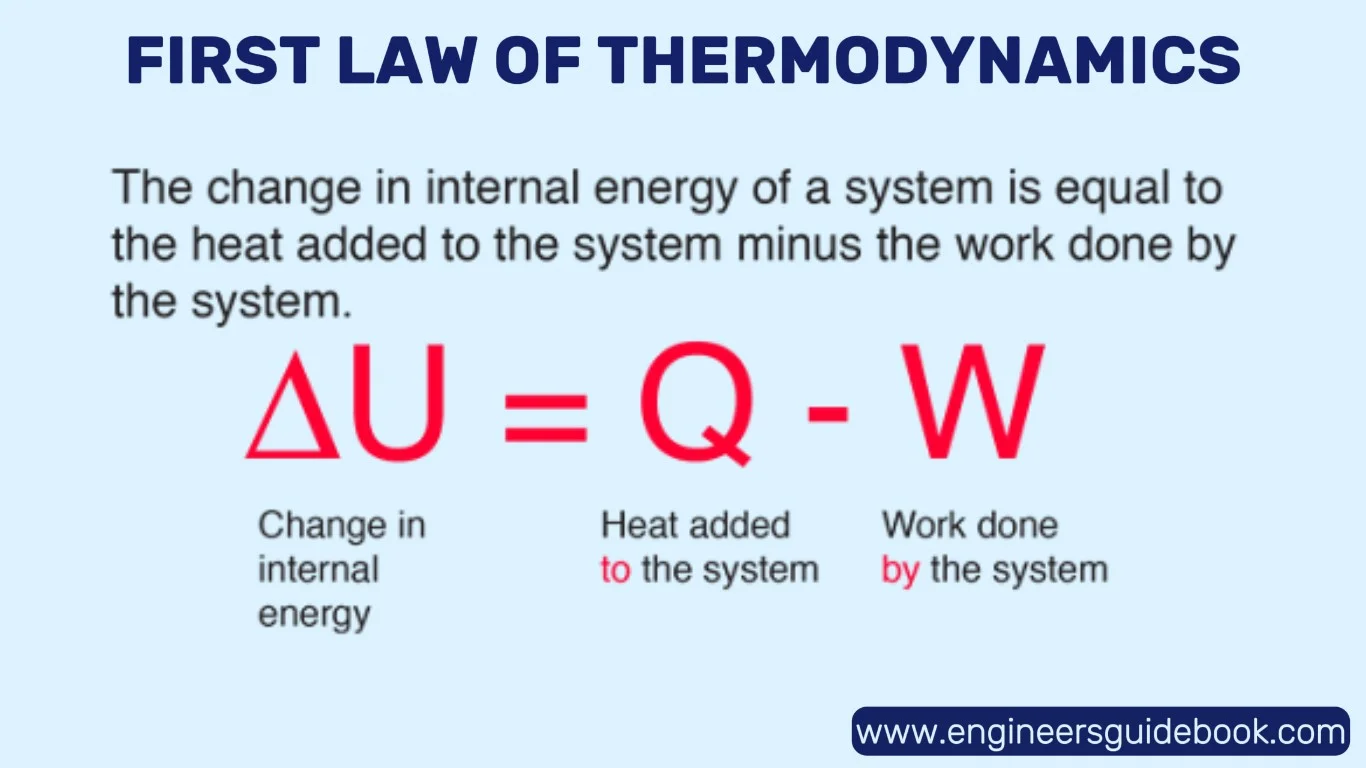
3.1 The Equation: ΔU = Q – W Explained
he First Law is encapsulated mathematically as:
ΔU = Q – W
- ΔU is the change in internal energy of the system
- Q is the heat added to the system
- W is the work done by the system
This compact formula offers a powerful tool for analyzing energy dynamics. It reveals that any increase in internal energy comes from either adding heat or reducing the work done by the system on its surroundings.
3.2 Real-World Interpretation of Each Term in the Formula
- ΔU (Internal Energy Change): Reflects how the system’s energy store changes over time. In practical terms, this could mean the heating of gas in a cylinder or cooling of a chemical reactor.
- Q (Heat Added): Represents energy entering the system due to temperature difference. It could be from a flame, friction, or even electrical resistance.
- W (Work Done): This refers to energy leaving the system when it performs work—like pushing a piston, spinning a turbine, or lifting a weight.
4. Energy Can Neither Be Created Nor Destroyed
4.1 The Philosophical and Physical Meaning
The idea that energy is eternal and immutable stretches beyond physics—it’s a philosophical axiom that challenges our perception of permanence and transformation.
In physics, this tenet manifests as the absolute constancy of energy in an isolated system. Regardless of how chaotic a process appears, the sum total of energy before and after remains identical.
It’s a profound reassurance in a world of constant flux.
4.2 Energy Transfer vs. Energy Transformation
Energy transfer refers to the movement of energy from one body or system to another, like heat passing from a hot cup to your hands.
Energy transformation, on the other hand, involves changing energy from one form to another—such as converting chemical energy in fuel into kinetic energy in an engine.
While the forms and locations of energy change, the quantity remains steadfast, reinforcing the universality of the First Law.
5. Understanding Heat (Q) and Work (W)
5.1 What Is Heat in Thermodynamics?
In thermodynamics, heat is not merely a sensation—it is energy in transit due to a temperature gradient. It flows spontaneously from hotter regions to cooler ones until thermal equilibrium is reached.
Unlike internal energy, heat is not stored within a system. Instead, it only exists as it crosses boundaries. This transient nature makes it a path function, dependent on the process, not just the end state.
5.2 Different Forms of Work
Work in thermodynamics transcends simple mechanics.
- Mechanical Work includes actions like rotating a shaft or moving a piston.
- Electrical Work involves energy transferred by electric currents, as in batteries or resistors.
- Pressure-Volume Work, perhaps the most iconic, occurs when gases expand or compress inside cylinders.
Each form of work alters the internal energy of a system, interacting dynamically with heat to define the system’s energetic journey. Recognizing these distinctions is essential for precise energy accounting in engineering and scientific analysis.
6. Internal Energy and Its Dependence on State
6.1 State Functions vs. Path Functions
In thermodynamics, state functions are properties that depend solely on the current condition—or state—of a system, irrespective of how it arrived there. Internal energy is a prime example. Whether a gas was heated slowly or compressed rapidly, its internal energy is defined by measurable variables like temperature, pressure, and volume.
Path functions, in contrast, are dependent on the specific process taken. Heat and work fall into this category. Their values change depending on the trajectory the system follows between two states. This distinction is pivotal: it underscores that internal energy is intrinsic to the system, while heat and work are transient, process-dependent phenomena.
6.2 Role of Internal Energy in Closed and Open Systems
In closed systems, internal energy changes due to heat exchange or work done, without any mass transfer. Think of a sealed piston—energy may enter or leave, but the matter remains contained.
Open systems, like steam turbines or human lungs, allow both energy and mass to cross boundaries. Here, internal energy is more dynamic, influenced not just by external work or heat but also by the enthalpy and kinetic energy of incoming and outgoing matter.
7. Closed, Open, and Isolated Systems
7.1 How the First Law Applies Differently to Each System Type
- Closed Systems: Energy can cross the boundary, but matter cannot. The First Law is applied to track energy changes via heat and work only.
- Open Systems: Both matter and energy can cross. The First Law adapts to include enthalpy flows and mass transport energy.
- Isolated Systems: Neither energy nor matter enters or exits. Here, the First Law simplifies—total energy remains eternally constant.
Each system type provides a unique lens to view energy conservation, making the First Law versatile across various physical scenarios.
7.2 Examples to Illustrate Energy Flow in Each System
- Closed System: A gas cylinder with a movable piston. Energy enters as heat, causing expansion and mechanical work.
- Open System: A boiling kettle. Water molecules escape as steam, carrying both mass and energy into the air.
- Isolated System: A thermos bottle in deep space. It neither absorbs nor emits significant energy, idealizing energy constancy.
8. Cyclic Processes and the First Law
8.1 Energy Conservation in Thermodynamic Cycles
Thermodynamic cycles like the Carnot or Rankine cycles are cornerstone models in engineering. These processes return a system to its original state, yet energy flows in and out continuously.
Despite intricate transformations, the net change in internal energy over a complete cycle is always zero.
8.2 Why Net Internal Energy Change Is Zero Over a Cycle
Because the system ends where it begins, the state variables—like temperature, pressure, and volume—are unchanged. Therefore, ΔU = 0.
However, heat and work interactions persist, often converting heat into mechanical work. This underscores that while internal energy resets, energy conversions still occur.
9. Applications of the First Law in Real Life
9.1 Engines, Refrigerators, and Air Conditioners
- Engines: Convert chemical energy into mechanical work using combustion. The First Law governs how much work can be extracted per unit of fuel.
- Refrigerators: Use work input to move heat against the temperature gradient. Energy is conserved, but in reverse fashion.
- Air Conditioners: Remove heat from interiors using mechanical compression. All devices rely on manipulating internal energy via heat and work.
9.2 Energy Management in Industrial
In industrial ecosystems, the First Law enables efficient energy tracking and cost optimization. Boilers, turbines, and heat exchangers operate based on energy conservation principles.
Power plants, for instance, maximize fuel-to-electricity conversion by meticulously accounting for heat losses and mechanical outputs.
11. Limitations of the First Law of Thermodynamics
11.1 Why It Doesn’t Address Directionality or Entropy
The First Law states that energy is conserved, but it says nothing about how energy is distributed or in what direction it flows. It doesn’t explain why heat flows from hot to cold, or why spontaneous processes occur.
11.2 How It Differs from the Second and Third Laws
- The Second Law introduces entropy and irreversible processes.
- The Third Law discusses the unattainability of absolute zero temperature.
The First Law sets the stage, but doesn’t narrate the full thermodynamic story.
12. Experimental Verification and Demonstrations
12.1 Laboratory Setups That Prove the First Law
Calorimetry experiments, gas compression chambers, and electrical heating coils all provide measurable insights into energy exchanges.
By monitoring heat and work, and comparing them with changes in internal energy, one can validate the First Law empirically.
12.2 Classic Experiments That Cemented the Law’s Status
James Joule’s paddle-wheel experiment stands as a historic testament. By stirring water with falling weights and measuring the resultant temperature rise, Joule demonstrated that mechanical work could be precisely converted into heat, thereby quantifying energy conservation.
15. Conclusion
The First Law of Thermodynamics is more than a scientific axiom—it is a universal truth. It governs all energetic interactions, ensuring balance in natural and engineered systems.
Energy may shift forms, move locations, or oscillate between states, but it never vanishes.
As humanity faces mounting energy challenges, thermodynamic principles will shape innovation. Advances in quantum thermodynamics, battery technologies, and zero-energy architecture all trace their lineage to the First Law.